Pulmonary manifestations of Q fever: analysis of 38 patients
Introduction
Q fever is caused by Coxiella burnetii, which can result in both acute and chronic manifestations. Lung involvement is rare, but is more often seen in acute Q fever (1-3). Symptoms can last from 10–90 days (4) and are more common in adults and in men (5). A study from Spain found that 65% of those with acute Q fever had respiratory symptoms (2). Pneumonia in these cases often present as non-productive cough, and fever (4). These patients have extrapulmonary manifestations as well including severe headache, myalgias, and arthralgias. Some patients can even present in acute respiratory distress. One study found nonspecific radiographic findings with similar appearance to those associated with pneumonia caused by viruses, mycoplasma pneumoniae, or chlamydia pneumoniae, or presenting as multiple rounded opacities (4). There have been a few reported cases of pseudotumor of the lung (6-8) and pulmonary fibrosis in chronic Q fever (9). Various studies have looked specifically at the radiographic findings with differing results, but these studies were done over 15 years ago (2,10,11).
Because of the nonspecific symptoms and various clinical presentations, there is often a delay in the diagnosis of Q fever, which may result in increased patient morbidity and mortality. The pulmonary manifestations of Q fever, specifically respiratory symptomology and thoracic radiographic findings, remain unclear especially in more recent years. The aim of this retrospective study is to provide a more updated review of the pulmonary presentation of Q fever, both acute and chronic.
Methods
We conducted a retrospective cohort study of patients with a diagnosis of Q fever between July 1, 2001 and December 31, 2014 at Mayo Clinic Rochester. This study was approved by the Mayo Institutional Review Board (#14-008728).
Diagnosis
We used a computer-assisted database search of clinical notes for a diagnosis of Q fever using the following terms ‘Q fever’ or ‘Coxiella burnetii’. A total of 69 patients were identified during this time period. Inclusion criteria included age ≥18 years, thoracic imaging around the time of diagnosis, and a confirmed diagnosis of either acute or chronic Q fever as based on the following: clinical diagnosis as per history and documentation AND C. burnetii phase I or II antigen titer >1:16 IgG (in serum), or positive C. burnetii polymerase chain reaction (in serum or tissue), or positive culture. Acute Q fever was suggested by a high IgG phase II titer and chronic Q fever by a high IgG phase I titer.
Data collection
Chart review was completed assessing demographics, co-morbidities, exposure history, risk factors for Q fever, symptoms and radiographic findings at time of diagnosis. A board certified chest radiologist (DW) reviewed all imaging of the chest including chest radiographs, computerized tomography (CT) chest, and positron emission tomography (PET) scan at time of diagnosis of Q fever. Based on clinical history, study investigators (DJ Kelm, DB White, M Baqir) determined if imaging could be correlated to Q fever. In cases that were not clear, a consensus was reached amongst the study investigators.
Statistical analysis
Descriptive analysis was conducted using JMP (version 10.0) software. Median and interquartile range (IQR) for continuous variables and frequency and percentage for categorical variables were used to summarize the data. To compare differences between acute versus chronic Q fever, a chi-square test was performed. P valued ≤0.05 were considered statistically significant.
Results
A total of 69 patients were initially identified between 2001 and 2014. Thirty-one patients were excluded: 3 pediatric patients, 20 patients did not meet serologic criteria for Q fever and 8 patients did not have imaging available from the time of initial diagnosis. We analyzed the remaining 38 patients.
Demographics
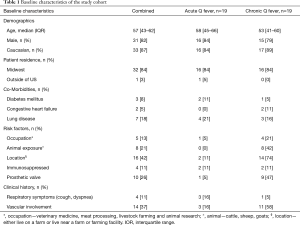
Table 1 describes the baseline characteristics of this study cohort. The median age was 57 years (IQR 43, 62) with 82% males. Thirty-two patients (84%) were from the Midwest. Nineteen cases (50%) had a diagnosis of acute Q fever. Four (21%) of those progressed to chronic Q fever.
Full table
History and clinical presentation
In this cohort, 4 (11%) were immunocompromised and 10 (28%) had a prosthetic heart valve. Those with a prosthetic heart valve were more likely to develop chronic Q fever (P=0.005). Most had nonspecific symptoms such as fevers (61%), chills (18%), and night sweats (21%). Acute respiratory symptoms were noted in 4 patients (11%), which consisted predominantly of cough, one of which also had hemoptysis. Respiratory symptoms were more commonly seen in acute Q fever (P=0.004). One patient with chronic Q fever presented with a lower extremity rash that was biopsied which was consistent with leukocytoclastic vasculitis.
Five patients (13%) had an occupation in which they had significant animal exposure, such as veterinary medicine, livestock farming and zoo keeper. Four (11%) were in close proximity or were involved with animal birthing. Another 5 patients (13%) had significant animal exposure to sheep, cattle, and/or goats. Sixteen patients (42%) lived on a farm, lived near a farm or farming facility, or had recently been on a farm; the majority of these patients had chronic Q fever. In total, 26 patients (68%) had potential exposure to animal reservoir of Q fever.
Radiographic findings
In this cohort, chest radiographs were obtained around the time of diagnosis in 30 patients (79%), 16 acute and 14 chronic Q fever. A CT chest was obtained in 22 patients (58%)—9 in acute and 13 in chronic. There were 9 total PET scans obtained (24%), 4 in acute and 3 in chronic.
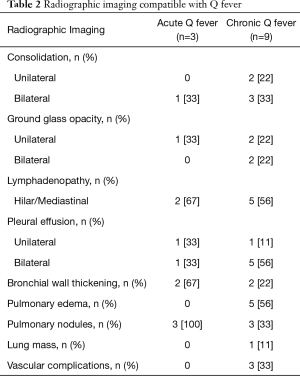
The main radiographic findings seen on chest radiographs or CT chest were consolidation (unilateral < bilateral), ground-glass opacities (unilateral > bilateral), pleural effusions (unilateral < bilateral), lymphadenopathy (hilar < mediastinal), pulmonary edema, and nonspecific pulmonary nodules. All those with consolidation were non-segmental.
Of these, 12 patients (32%) had imaging that was thought to be attributable to Q fever—3 (25%) with acute Q fever and 9 (75%) with chronic Q fever (Table 2). The right upper lobe mass-like consolidation (Figure 1), seen in a patient with chronic Q fever, was biopsied via CT-guidance with the pathology significant for lymphoplasmacytic infiltration in the background of fibrosis. No patients in this study were found to have findings of pulmonary fibrosis attributable to Q fever.
Full table
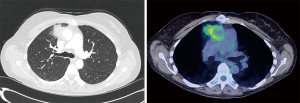
The findings noted on PET scan in those with chronic Q fever included fluorodeoxyglucose (FDG) avidity along a vascular graft and increased FDG accumulation in the soft tissue thickening along the ascending aorta, contiguous medial right upper lobe consolidation, and manubrial erosion (Figure 1). For acute Q fever, there was one patient with FDG avid thoracic and abdominal lymphadenopathy that was biopsy negative for malignancy and consistent with a granulomatous process (without findings of a fibrin ring or “doughnut” granuloma seen in Q fever) with hepatic and bone lesions presumed to be secondary to melanoma metastases.
Treatment and outcomes
The management of Q fever depended on whether it was acute or chronic, but often included either doxycycline alone or a combination of doxycycline and hydroxychloroquine. In those with chronic Q fever, surgical intervention was required in 8 cases (21%); the type of intervention depended on the specific involvement of Q fever: 2 aortic root replacements/repair; 2 aortic valve replacements; 3 spinal debridements. Those with imaging findings consistent with chronic Q fever were more likely to have underwent surgical intervention (P=0.0006).
In this cohort, there were 3 deaths (8%); one of which was attributed to chronic Q fever with endocarditis, mycotic aneurysm and vertebral infection. The cause of death in the other 2 cases is unknown, but both had chronic Q fever (one with aortitis and graft infection and the other with endocarditis).
Discussion
Our study found that pulmonary manifestations were uncommon in Q fever but included cough in acute Q fever and imaging findings of vascular complications, pulmonary edema with pleural effusions, and consolidation were more likely to be seen in chronic Q fever.
The demographics noted in our study cohort are similar to prior studies. Specifically, we had a male predominance as seen in France with a male/female ratio of 2.45 for acute Q fever (12). Men are more likely to be engaged in occupations associated with risk for development of Q fever. Additionally, as based on mouse models, the gender difference could be related to hormones in which estradiol may play a protective role (13). Exposure has been linked to parturient cats and rabbits, sheep and cattle, and ingestion of contaminated milk products (14,15). It is more likely that inhalation of infected aerosolized particles results in transmission due to its hypothesized sporulation process (16). In our cohort, 10 patients were directly in contact with animals either by their job or for other reasons. Many either lived on a farm or lived close to a farm. Even without known direct contact, transmission is thought to occur through the wind as a prior study has found only 25% of those that developed Q fever to have direct animal exposure (17), which is similar to our study cohort as 32% did not have any known exposure history to suggest Q fever.
Acute and chronic Q fever has various clinical presentations. Acute Q fever is often asymptomatic (18); if there are symptoms, it is often a self-limited febrile illness with headaches. Lung involvement is often nonspecific and similar in appearance to those associated with atypical pneumonia (2,18-20). The most common clinical presentation in our study was fever with only a few cases of cough. Chronic Q fever is defined as duration of infection for more than 6 months; it occurs in approximately 1–5% of those infected with C. burnetii (4). Pregnant females, immunocompromised hosts, and those with underlying valvular or vascular disease are at an increased risk for development of chronic Q fever. Our study also found that prosthetic valve resulted in a statistically significant risk for development of chronic versus acute Q fever. Presentation at this stage most commonly includes endocarditis, infection of aneurysms or vascular grafts and osteomyelitis (4). In our cohort, vascular complications included aortic pseudoaneurysm, graft infection, endocarditis, osteomyelitis, encephalitis, and prosthetic knee infection. Lung involvement in chronic Q fever is rare. In our study, there was one case of pulmonary pseudotumor contiguous with abnormal periaortic soft tissue in the setting of infected pseudoaneurysm. Prior case reports noted pseudotumor of the lung in acute Q fever (6,7,9) and more recently this has been described in chronic Q fever (8). Additionally, chronic pulmonary fibrosis has also been reported (9,21) though not seen in our study.
Prior studies evaluating the radiographic findings of Q fever found 75% had lung consolidation (lower lobe predominance) (2) and that the most common abnormalities were unilateral, single-segmental opacities, often in the upper lobes (10). Lobar opacities were less common. The findings of acute Q fever pneumonia on CT chest has been found to be multi-lobar airspace consolidation with a nodular pattern with surrounding halo of ground-glass opacification and necrotizing pneumonia less common (11). Pleural effusions and mild lymph node enlargement were also noted (11). In our study, findings of acute Q fever included consolidation, ground-glass opacity, pleural effusions, nonspecific pulmonary nodules, and reactive lymphadenopathy. Those with chronic Q fever were more likely to have imaging findings related to edema, pleural effusions, or cardiovascular complications. The imaging findings of Q fever are nonspecific; however chest radiography and CT may demonstrate important findings in symptomatic patients especially with respiratory symptomatology. Chest CT or CT angiography is particularly helpful in patients with suspected cardiovascular complications in the setting of chronic Q fever. The role of PET scan remains unclear but may have utility in those patients with chronic Q fever and a known cardiac valve or vascular surgical history.
The limitations of this study relate to the small sample size and inherent flaws of a retrospective study including selection bias. The strengths include the long time frame reviewed and the inclusion of various thoracic radiology modalities. The results of this study provides the pulmonologist a better understanding of the underlying respiratory symptoms and radiographic findings to be considered in the diagnosis of both acute and chronic Q fever and especially in those cases in which the diagnosis remains unclear. To better understand these uncommon pulmonary findings of Q fever would require future research involving multiple institutions across the world due to the various geographic locations of this zoonotic infection and include a long time frame of review due to its rarity.
In conclusion, pulmonary symptomatology and chest imaging findings such as consolidation, lymphadenopathy, or lung mass seen with Q fever is uncommon, but can occur in chronic Q fever and should be considered in the appropriate clinical context.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work was presented as a poster at CHEST 2015 in Montreal on October 28th, 2015.
Ethical Statement: This study was approved by the Mayo Institutional Review Board (#14-008728).
References
- Marrie TJ. Q fever, 1979-1987--Nova Scotia. Can Dis Wkly Rep 1988;14:69-70. [PubMed]
- Montejo Baranda M, Corral Carranceja J, Aguirre Errasti C. Q fever in the Basque Country: 1981-1984. Rev Infect Dis 1985;7:700-1. [Crossref] [PubMed]
- Tselentis Y, Gikas A, Kofteridis D, et al. Q fever in the Greek Island of Crete: epidemiologic, clinical, and therapeutic data from 98 cases. Clin Infect Dis 1995;20:1311-6. [Crossref] [PubMed]
- Raoult D, Marrie T. Q fever. Clin Infect Dis 1995;20:489-95. [Crossref] [PubMed]
- Tissot-Dupont H, Vaillant V, Rey S, et al. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 2007;44:232-7. [Crossref] [PubMed]
- Janigan DT, Marrie TJ. An inflammatory pseudotumor of the lung in Q fever pneumonia. N Engl J Med 1983;308:86-8. [Crossref] [PubMed]
- Lipton JH, Fong TC, Gill MJ, et al. Q fever inflammatory pseudotumor of the lung. Chest 1987;92:756-7. [Crossref] [PubMed]
- Polo MF, Mastandrea S, Santoru L, et al. Pulmonary inflammatory pseudotumor due to Coxiella burnetii. Case report and literature review. Microbes Infect 2015;17:795-8. [Crossref] [PubMed]
- Brouqui P, Dupont HT, Drancourt M, et al. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch Intern Med 1993;153:642-8. [Crossref] [PubMed]
- Gikas A, Kofteridis D, Bouros D, et al. Q fever pneumonia: appearance on chest radiographs. Radiology 1999;210:339-43. [Crossref] [PubMed]
- Voloudaki AE, Kofteridis DP, Tritou IN, et al. Q fever pneumonia: CT findings. Radiology 2000;215:880-3. [Crossref] [PubMed]
- Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985-1998. Clinical and epidemiologic features of 1,383 infections. Medicine 2000;79:109-23. [Crossref] [PubMed]
- Leone M, Honstettre A, Lepidi H, et al. Effect of sex on Coxiella burnetii infection: protective role of 17beta-estradiol. J Infect Dis 2004;189:339-45. [Crossref] [PubMed]
- Langley JM, Marrie TJ, Covert A, et al. Poker players' pneumonia. An urban outbreak of Q fever following exposure to a parturient cat. N Engl J Med 1988;319:354-6. [Crossref] [PubMed]
- Marrie TJ, Durant H, Williams JC, et al. Exposure to parturient cats: a risk factor for acquisition of Q fever in Maritime Canada. J Infect Dis 1988;158:101-8. [Crossref] [PubMed]
- McCaul TF, Williams JC. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol 1981;147:1063-76. [PubMed]
- Tissot-Dupont H, Torres S, Nezri M, et al. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol 1999;150:67-74. [Crossref] [PubMed]
- Dupuis G, Petite J, Peter O, et al. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol 1987;16:282-7. [Crossref] [PubMed]
- Marrie TJ. Coxiella burnetii (Q fever) pneumonia. Clin Infect Dis 1995;21 Suppl 3:S253-64. [Crossref] [PubMed]
- Marrie TJ, Pollak PT. Seroepidemiology of Q fever in Nova Scotia: evidence for age dependent cohorts and geographical distribution. Eur J Epidemiol 1995;11:47-54. [Crossref] [PubMed]
- Aitken ID, Bogel K, Cracea E, et al. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection 1987;15:323-7. [Crossref] [PubMed]


