Management of occult malignant pleural disease firstly detected at thoracotomy for non-small cell lung cancer patients
Introduction
Non-small cell lung cancer (NSCLC) patients with malignant pleural disease (MPD), which includes malignant pleural effusion (MPE) and/or malignant pleural nodules (MPN), are generally considered to be inoperable and the prognosis of this disease has remained persistently bad during the last 30 years (1-3). MPD is often discovered at thoracotomy for the NSCLC patients, and the treatment of primary cancer lesion is always controversial (4-8).
Therefore, we aimed to evaluate the risk factors for the occult MPD and find optimal prediction model to provide the appropriate multimodality therapy. We also studied how to treat primary lesion that could benefit for the outcome of NSCLC patients.
Methods
Patients
A total of 2,093 consecutive cases of NSCLC patients who underwent thoracotomy or video-assisted thoracic surgery from January 2006 to January 2015 in Peking University Cancer Hospital were involved in current study. Computed tomography (CT) scanning of the thorax, a bone scan, a CT or magnetic resonance imaging (MRI) scan of the head, and ultrasound scanning of the abdomen and supraclavicular lymph nodes or positron emission tomography (PET)/CT of whole body were conducted for the involved patients that ruled out the disease with distant metastasis. Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA), transesophageal endoscopic ultrasound guided fine needle aspiration (EUS-FNA) or mediastinoscopy were used to determine the N2 patients. To exclude clinical stage IV disease, all patients underwent full oncological staging before surgery. During surgery, 110 patients (5.26%) who had MPD including MPD with/without MPE were proven with pathological stage IV disease. These 110 cases were diagnosed as occult MPD. Disease stage was evaluated, according to the TNM Classification of Malignant Tumors, 7th Edition. The participants signed informed consent and this study has been approved with the Ethics Committee of Peking University Cancer Hospital.
Surgical procedures
All of the surgical resections and nodal dissections were conducted by thoracic surgeons at the department of Thoracic Surgery II of Peking University Cancer Hospital. There were 447 pathological N2 disease and 334 cases were occult N2. The 110 patients with occult MPD were divided into two groups with or without primary lesions resection. Forty eight patients were received exploratory thoracotomy (open-close surgery) with biopsy of metastasis focuses, and 62 patients were carried out palliative resection of lung including sub-lobectomy (n=44) and lobectomy (n=18).
Statistical analysis
Comparison among clinical characteristics including gender, age, imaging tumor size, carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199), neuron specific enolase (NSE), cytokeratin 19 fragment 21-1 (CYFRA21-1), squamous cell carcinoma (SCC) antigen, smoking status, neoadjuvant therapy, PET/CT scan, tumor location, clinical T (cT) stage, clinical node (cN) stage, differentiated degree, pathological type, pleural invasion, and vascular thrombosis were assessed using the non-parametric test (Mann-Whitney-U test) and χ2-test. Association between occult MPD and clinical values were prepared by Multivariate analysis (logistic regression-backwards stepwise method). In addition, not all 2093 patients were tested for tumor markers and the number of CEA, CA125, CA199, NSE, CYFRA21-1and SCC was 1,723, 1,228, 1,246, 1,700, 1,696 and 1,686, respectively.
Follow-up was taken from the statistical office of follow-up in Peking University Cancer Hospital and the cut-off date of follow-up for our study was 1 Dec. 2016. The terminal event was death from any cause. Survival curves were estimated by the Kaplan-Meier method, and significance was assessed by the log-rank test. P<0.05 was considered as statistically significant difference. All statistical calculations were performed using the SPSS Statistics 22.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Clinical characteristics and tumor biomarkers for the NSCLC patients with occult MPD
The clinical characteristics for the 2,093 cases of NSCLC patient are described in Table 1. There were 110 patients detected with MPD during thoracotomy. Younger age (P=0.001), higher serum CEA and CA125 level (P<0.001 & P=0.001), advanced N stage (P<0.001), adenocarcinoma (P<0.001), pleural invasion (P<0.001) and vascular thrombosis (P=0.001) were associated with occurrence of occult MPD.
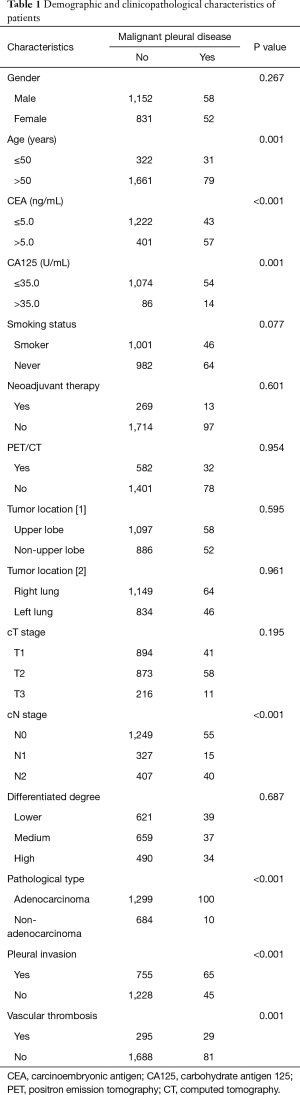
Full table
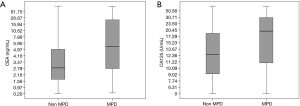
We analyzed the relationship between MPD and tumor biomarkers including CEA, CA125, CA199, NSE, CYFRA21-1 and SCC (Table 2). Higher CEA and CA125 levels were significantly correlated with occult MPD and P value equals to 0.003 and 0.012, respectively. 5.0 ng/mL and 35.0 U/mL were cut-off value for CEA and CA125 that were provided by clinical laboratory. The mean values ± standard deviation of CEA and CA125 were 10.7±67.2 vs. 31.8±74.6 ng/mL and 19.8±56.4 vs. 37.7±68.7 U/mL for the NSCLC patients without and with MPD, respectively (Figure 1A,B). However, there were no statistically significant differences in CA199, NSE, CYFRA21-1 and SCC level for the patients with or without MPD both groups. Interesting, smaller tumor size was related to occurrence of occult MPD, although there was no significant difference (P=0.704).
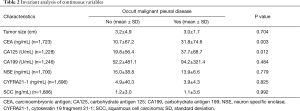
Full table
A predictive model to predict MPD for NSCLC
Next, we evaluated whether clinical features including gender, age, CEA, CA125, smoking status, N stage, pathologically type, pleural invasion and vascular thrombosis related to MPD by a logistic regression analysis. We found that age ≤50 (OR 1.781, 95% CI: 0.988–3.211, P=0.055), serum CEA level (OR 1.005, 95% CI: 1.001–1.008, P=0.006), advanced N stage (N2 vs. N0, OR 2.266, 95% CI: 1.286–3.990, P=0.005), adenocarcinoma (OR 7.232, 95% CI: 2.227–23.486, P=0.001) and pleural invasion (OR 1.709, 95% CI: 1.022–2.858, P=0.041) were independent risk factors for occult MPD (Table 3). The coefficients were also showed in Table 3.
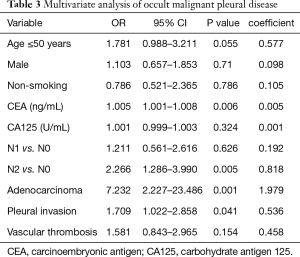
Full table
Thus, these five independent risk factors with coefficients assessment were remained in the final prediction model (Figure 2). A new predictive scoring system was then created as the following equation: risk score =age (≤50) ×0.577 + CEA (ng/mL) ×0.005 + N1 ×0.192/N2 ×0.818 + adenocarcinoma ×1.979 + pleural invasion ×0.536.
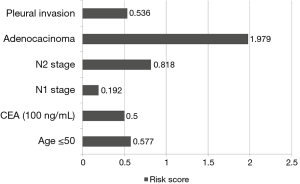
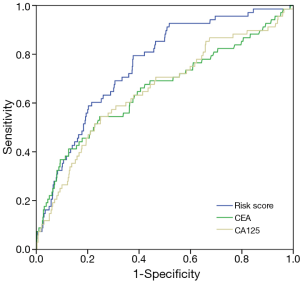
Figure 3 showed the receiver operating characteristic curve (ROC) analysis of serum CEA level, serum CA125 level and the risk score that suggesting that risk score was optimal prediction model for occult MPD with 0.756 of area under curve (AUC) (P<0.001). The AUC value for CEA and CA125 were 0.657 and 0.654. The sensitivity and specificity of risk score were 79.4% and 62.3% with the optimal cut-off value 2.795. The sensitivity and specificity of CEA were 54.4% and 75.3% with cut-off value 4.78 (ng/mL), and CA125 were 54.4% and 75.0% with cut-off value 18.60 (U/mL).
Survival analysis
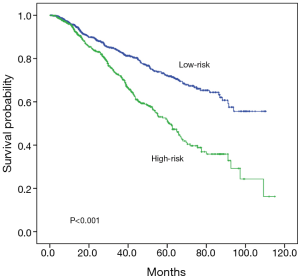
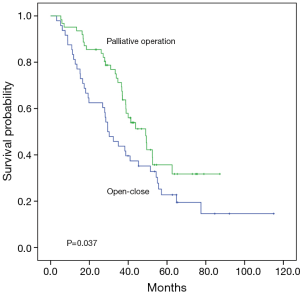
All 2,093 patients were then classified into a high-risk (risk score >2.795) and low-risk group (risk score ≤2.795) according to the cut-off value from the ROC curve analysis. Patients with a high-risk score had a significantly shorter median survival time (61.4 months vs. not reached) and lower 5-year survival rate (51.1% vs. 71.8%) with significant difference (P<0.001 for both, Figure 4). The median survival time, the 3-year survival and 5-year survival rate for the patients without and with MPD and MPD were 91.5 months, 79%, 67% and 38.9 months, 58%, 28%, respectively (P<0.001). The survival curve for the 110 MPD patients demonstrated there was a better prognosis for the patients whose primary lung cancer were removed than the patients who only suffered open-close surgery (median survival time 49.0 vs. 29.4 months, 3-year survival 69.4% vs. 41.7%, 5-year survival 31.7% vs. 19.5%, P=0.037, Figure 5).
Discussion
NSCLC patients with occult MPD, characterized by MPE and/or MPN, are reported to have poor prognosis. According to the seventh edition of the UICC lung cancer staging system, the disease of MPE and/or MPN is considered as stage IV (M1a). The IASLC Lung Cancer Staging Project reported that patients with clinical MPD had a dismal prognosis, with an median survival time of 8 months and a 2% 5-year survival rate (3). MPD patients with clinical diagnosis generally present with a fair amount of MPE, relatively less MPD patients detected during thoracotomy have effusion or pleural nodules (6,9). Our study indicated the rate of occult MPD was 5.26% and the 5-year estimated survival rate was 28% that were consistent to Okamoto’s research (8).
In the present study, we found that higher serum CEA and CA125 levels were significantly correlated with MPD. CEA is a glycoprotein of molecular weight 180KD and is a tumor marker to have relatively high sensitivity for advanced adenocarcinomas. Serum CEA concentrations are higher in adenocarcinoma and large cell carcinoma (10-12). CA125 is released from the pleura as well as the peritoneum and the positivity of this marker was recognized in the cytosolic materials of pleural effusion sediments and no significant difference was observed between benign and malignant diseases (13). Based on the previous reports (14,15), CA125 is not recommended as a useful diagnostic tool in MPE. In present study, serum CA125 level was significantly higher in the patients with occult MPD, although there was no significant difference by the multivariate analysis. Additionally, in view of the association between CA125 and pleural effusions, this biomarker was incorporated into ROC analysis as comparison of integrated prediction model.
Since few studies were done to predict occult MPD for the NSCLC patients, we analyzed clinical values and tumor markers to obtain diagnostic utility by multivariate analysis. The integrated prediction model of evaluating risk score consisted of age, CEA, N stage, pathological type and pleural invasion showed better diagnostic efficiency with AUC 0.756. According to this model, the outcome for the NSCLC patients with high-risk score was obviously worse than the patients with low-risk score. We may design a clinical trial to evaluate the survival benefit for the patient with MPD and it could be suggested that the patients with high-risk score could considered to re-stage and receive neoadjuvant treatment, which is the clinical implication of predictive scoring model.
When MPD is detected during thoracotomy, the treatment for the primary tumor is controversial. Generally, patients with minimal pleural dissemination detected on lavage cytology have been subjected to surgery. It is also controversial that the treatment of patients with MPD plus sufficient effusion for standard cytological examination or macroscopically detected pleural nodules at thoracotomy (16,17). The survival data from the IASLC Staging Project indicated patients pathologically diagnosed with MPD had with 18 months of median survival time and 11% of 5-year survival rate, showing better prognosis than the patients with clinical diagnosis of MPD (3,18). In this study, we assessed the relevance of pulmonary resection for the clinical stage I-III patients with MPD detected during thoracotomy. In consistent to the previous studies (19), the NSCLC patients with occult MPD had poor outcome than the patients diagnosed as Stage I-III disease without MPD.
Among 110 patients with occult MPD, 62 patients (56.4%) were subjected to palliative primary tumor resection including 44 sub-lobectomies and 18 lobectomies. And the outcomes including median survival time, 3-year survival and 5-year survival analyses for the patients with primary tumor resection were better than the patients with open-close surgery that indicated that primary lesion resection could promote survival for the MPD NSCLC patients. An previous Japanese study suggested the pleural carcinomatosis accounted for 2.9% and macroscopic complete resection for them was associated with better 5-year survival rate with 37.1% (20). Our institution multidisciplinary team of thoracic tumor had an agreement for the NSCLC patients with MPD firstly observed during thoracotomy should accept primary lesion resection if patients could tolerate surgical injury. In addition, patients with preoperatively suspected MPD should accept neoadjuvant therapy and then stage be reappraised.
However, these analyses were not randomized studies that could ignore the selection bias for determining the treatment for the primary lesion. Those patients with more peripheral, small or negative lymph node and better performance status were greatly recommend to proceed with resection. A retrospective review and the issue about postoperative evaluation of pleural invasion and vascular thrombosis rather than clinical image feature before surgery are limited. In future study, we will improve these drawbacks to acquire more compelling data.
In sum, the occult MPD for the NSCLC patients detected during operation and association between the occult MPD and young age, high serum CEA level, advanced N stage, adenocarcinoma or pleural invasion was better to be concerned by all the clinician. Our new prediction model of risk score for the patients’ evaluation could help doctors to determine optimal multimodality therapy. Primary lesion resection is a more suitable option to improve survival rate when encountering occult MPD during the operation instead of exploratory thoracotomy.
Acknowledgements
We thank Servbus Technology (Beijing) Co., Ltd for the database support and statistical analysis.
Funding: This work was supported by the Capital Health Research and Development of Special funding (No. 2014-2-1021) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special funding (No. ZYLX201509).
Footnote
Conflicts of Interest: The authors have no conflicts of interests to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Peking University Cancer Hospital. In addition, the Number of Ethic Approval was 2015KT04. The participants signed informed consent before thoracotomy which included a statement to approve their disease information could be used in this study.
References
- Sugiura S, Ando Y, Minami H, et al. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 1997;3:47-50. [PubMed]
- Jett JR, Schild SE, Keith RL, et al. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:266S-276S.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Ichinose Y, Tsuchiya R, Koike T, et al. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today 2000;30:1062-6. [Crossref] [PubMed]
- Ohta Y, Tanaka Y, Hara T, et al. Clinicopathological and biological assessment of lung cancers with pleural dissemination. Ann Thorac Surg 2000;69:1025-9. [Crossref] [PubMed]
- Fukuse T, Hirata T, Tanaka F, et al. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer 2001;34:75-81. [Crossref] [PubMed]
- Shiba M, Kakizawa K, Kohno H, et al. Prognostic implication of Ki-67 immunostaining in treating subclinical pleural cancer found at thoracotomy in lung cancer patients. Ann Thorac Surg 2001;71:1765-71. [Crossref] [PubMed]
- Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [PubMed]
- Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc 2008;83:235-50. [Crossref] [PubMed]
- Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 2003;123:105S-114S. [Crossref] [PubMed]
- Wagner IC, Guimaraes MJ, da Silva LK, et al. Evaluation of serum and pleural levels of the tumor markers CEA, CYFRA21-1 and CA 15-3 in patients with pleural effusion. J Bras Pneumol 2007;33:185-91. [Crossref] [PubMed]
- Sharma SK, Bhat S, Chandel V, et al. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, and cytokeratin 19 fragments in patients with effusion from nonsmall cell lung cancer. J Carcinog 2015;14:7. [Crossref] [PubMed]
- Terracciano D, Mazzarella C, Cicalese M, et al. Diagnostic value of carbohydrate antigens in supernatants and sediments of pleural effusions. Oncol Lett 2010;1:465-71. [PubMed]
- Ghayumi SM, Mehrabi S, Doroudchi M, et al. Diagnostic value of tumor markers for differentiating malignant and benign pleural effusions of Iranian patients. Pathol Oncol Res 2005;11:236-41. [Crossref] [PubMed]
- Shitrit D, Zingerman B, Shitrit AB, et al. Diagnostic value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literature. Oncologist 2005;10:501-7. [Crossref] [PubMed]
- Sawabata N, Matsumura A, Motohiro A, et al. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: is tumor resection beneficial for prognosis? Ann Thorac Surg 2002;73:412-5. [Crossref] [PubMed]
- Mordant P, Rivera C, Legras A, et al. Current readings: the most influential and recent studies regarding resection of lung cancer in m1a disease. Semin Thorac Cardiovasc Surg 2013;25:251-5. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Vicidomini G, Santini M, Fiorello A, et al. Intraoperative pleural lavage: is it a valid prognostic factor in lung cancer? Ann Thorac Surg 2005;79:254-7; discussion 254-7. [Crossref] [PubMed]
- Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. [Crossref] [PubMed]


