Cytokeratin-based CTC counting unrelated to clinical follow up
Introduction
Circulating Tumor Cells (CTCs) represent a biomarker which is also potentially involved in development of metastasis, thus their identification and characterization may bring hope for improved follow up in patients with cancer. However, despite a relevant number of studies and investments aimed to change the clinical practice and improve patients’ follow up through the use of this new marker, the clinical benefit of CTC is still under debate. A major role in this setting is played by the method used to extract CTC from blood, as we know now that CTC are not only extremely rare but also heterogeneous, including CTC with epithelial markers, with mesenchymal markers, and CTC in epithelial to mesenchymal transition (EMT) having both types of markers. Thus, it is conceivable that methods using CTC capture based on epithelial markers, such as cytokeratins (CKs) and EpCAM, may lose the most malignant mesenchymal CTCs thus decreasing the clinical value of the whole test. In this context, we have assessed two different approaches for CTC detection, one “CK-based” and the other antigen-independent “cell size-based”, according to the clinical follow up in a patient with metastatic lung cancer.
Lung cancer is a common cause of cancer death worldwide, expected to account for 26% of all female cancer deaths and 29% of all male cancer deaths (1). Despite advances in early detection and treatment, non-small-cell lung cancer (NSCLC) is often diagnosed at an advanced stage and has a poor prognosis. In this context, a reliable test for counting and analysis of CTCs, believed to be crucial in cancer dissemination and metastasis, could represent a tool with prognostic and predictive significance, also providing a “liquid” biopsy for personalized anticancer therapy.
The aim of this work was to perform a kinetic study of CTCs in a patient with metastatic undifferentiated NSCLC, assessing two non-automated techniques for CTC detection according to the patient’s follow up and cancer evolution.
Materials and methods
Patient
For CTC analysis, blood samples (2×8 mL) were collected from a patient with metastatic undifferentiated NSCLC at the A.C. Camargo Cancer Hospital in São Paulo, Brazil, after written, informed consent from patient and approval by the Local Research Ethics Committee (protocol number: 1367-10). Patient clinical-pathological parameters were obtained from the medical records.
Samples were collected in EDTA tubes following time schedule shown in Table 1. After blood collection, tubes were stored at room temperature for subsequent analysis by Carcinoma Cell Enrichment and Detection kit with MACS technology (MiltenyiBiotec, BergischGladbach, Germany) within 24 hours, according to information on data sheet (Miltenyi Biotec, catalog number 030-060-101) (8 mL of blood) and by isolation by size of epithelial tumor cells (ISET) (Rarecell Diagnostics, Paris, France) according to manufacturer instructions (8 mL of blood).
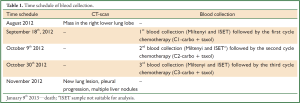
Full Table
CTC ENRICHMENT The Carcinoma Cell Enrichment and Detection kit with MACS technology selects CTCs by immunomagnetic separation with anti-pan CK antibody that recognizes CK 7, 8, 18 and 19, according to manufacturer procedures and methodology described by Nadal et al. (2). CTCs were defined by the following criteria: CK+ cell with high nucleo-cytoplasmic ratio and larger than leukocytes (3).
ISET methodology isolates intact CTCs from blood through direct filtration without using antibodies, thus exploiting the larger size of CTC as compared with leukocytes, and uses polycarbonate membrane with 8-μm-diameter cylindrical pores. We used procedures of this platform and CTC definition criteria according to previous detailed reports (4). Circulating tumor microemboli (CTM) were defined as groups or clusters of tumor cells containing three or more distinct nuclei and previously identified in metastatic cancer patients (5).
Images of results obtained with the two techniques were taken using a light microscope (Axioskop 40-Carl Zeiss, Germany) coupled to a digital camera (Sony Cyber-shot DSC-S75) at 100× magnification.
Both methods used here are for research use only. However, ISET underwent technical (6,7), and clinical validation (8) in particular, showing the prognostic value of ISET in patients with early and late stage Non Small Cells Lung Cancer.
Immunocytochemistry
Immunohistochemistry studies were feasible only on CTC isolated by ISET. ISET membrane spots were cut out and used for single or dual-color immunocytochemistry for CTC identification and characterization according to the manufacturer’s instructions.
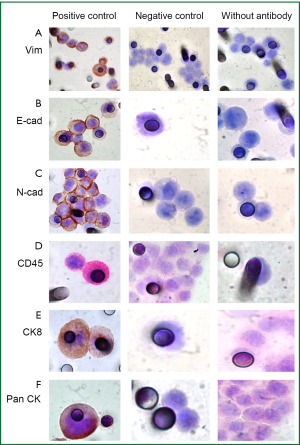
To evaluate and distinguish CTCs from White Blood Cells (WBCs) contaminants, besides morphology analysis, dual-color immunocytochemistry (DAB+/Permanent Red; DakoTM) was carried out using the following antibodies: anti-CK7 (1:50; clone OV-TL 12/30, DakoTM) and anti-CD45 (1:100; clone 2B11+ PD7/26, DakoTM), a WBC surface marker; and anti-CK8 (1:50; clone NCL-L-CK8-TS1, NovocastraTM). A dual-color immunocytochemistry approach was also used to evaluate EMT on CTCs. The antibodies used were anti- Pan CK (1:1,000; clone AE1/AE3, Dako), anti-E-cadherin (1:100; clone 36, BD Bioscience) and anti-vimentin (1:1,500; clone V9, Dako).
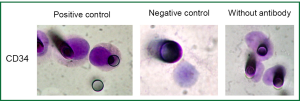
Single immunocytochemistry (DAB+/DakoTM) was performed to evaluate N-Cadherin expression (1:50; clone 6G11; DakoTM), a marker of EMT and CD34 expression, a marker of endothelial cells (1:100; clone QBEnd 10, DakoTM).
Negative and positive controls were performed for each IHC staining (Figures S1,S2).
These tests were performed at baseline blood collection (before first cycle of chemotherapy) and before the third cycle of chemotherapy. For CTCs counting, eight spots were used (corresponding to 8 mL of blood) including 4 stained with hematoxylin-eosin and the others stained by ICC as described by Krebs et al. (7).
Results
A 68-year-old woman presented in August 2012 with a history of persistent chest pain in the right hemithorax and a lump measuring about 3 cm in the right inframammary region.
Computed Tomography (CT) scan of the chest showed a cavitated mass approximately 8.7 cm × 7 cm, in the right lower lung lobe, with pleural effusion and nodular thickening in the mid-basal portion of the pleura, and infiltration of 6th right anterior rib. Furthermore, enlarged paratracheal and infracarinal lymph nodes (approximately 1.6 cm), paraseptal and centrilobular emphysema as well as basal ground-glass opacities were found.
Subsequently, a biopsy of the chest wall was carried out and revealed undifferentiated NSCLC. Immunostaining was positive for Pan CK AE1/AE3, CK7 and p63 (focally) and negative for CK20, TTF-1, calretinin, napsin A, CD31, CD34, S-100 and FLI-1. Positron emission tomography-CT (PET-CT) revealed stage IV disease (T4, N2, M1; metastasis in non-regional lymph nodes). It showed paratracheal, fracarinal and periesofagic lymphadenopathy.
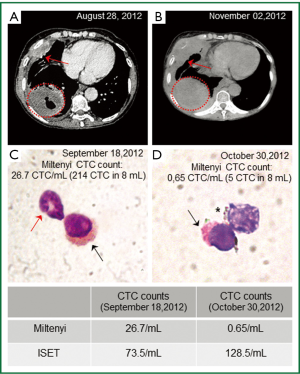
Concerning the blood samples collected before the first cycle of chemotherapy (See Methods), the immunomagnetic separation (Miltenyi kit) showed 26.7 CTC/mL (total 214 in 8 mL) with high expression of CK (Figure 1); while the ISET approach showed 735/mL CTC (total estimated: 588 in 8 mL).
Carboplatin and paclitaxel treatment were initiated. After one cycle of chemotherapy, a new CTCs analysis was performed, but the blood sample for ISET was not suitable for analysis (because of coagulation due to lack of blood agitation after collection), thus we could perform the immunomagnetic separation (Miltenyi kit) method only which showed 13.7 CTC/mL (total 110 in 8 mL) with high expression of CK and morphology as in the first analysis, possibly suggesting a response to treatment. However, the number of CTCs was still high and could represent a potential minimal residual disease (9,10); Events that were observed during the time course of patient disease.
Despite the decreased number of CTC detected by Miltenyi, the imaging studies showed a progression of the disease. Figure 1A,B, shows that, although there was no significant difference in tumor size in a 4-month interval between diagnosis and the end of the third cycle of chemotherapy, a new lung lesion measuring 8 mm and pleural progression were identified, demonstrating disease progression according to Response Evaluation Criteria In Solid Tumors (RECIST) (11). This CT scan performed after the third cycle of chemotherapy (4-month interval since the discovery of the lung disease) also revealed progression of disease to liver (multiple nodules up to 3 cm) and retroperitoneal lymph nodes.
Before the third cycle of chemotherapy, we performed another CTCs analysis using the ISET platform, as well as with the immunomagnetic Miltenyi method. The immunomagnetic separation (Miltenyi kit) showed 0.65 CTC/mL (total 5 CTC in 8 mL) with heterogeneous expression of CK and a different expression pattern than the previous analysis (Figure 1D); While the ISET approach showed 128/mL CTC (total estimated 1,024 CTC in 8 mL) which was consistent with the clinical worsening of the disease (imaging progression). CTC morphology was similar by using both methods, although the counts per 8 mL of blood collection were different, the counts by ISET being consistently higher than the counts by immunomagnetic labeling (Miltenyi).
Results obtained by ISET before the third cycle of chemotherapy also demonstrated the presence of CTM (Figure 2). These CTMs have been reported to be associated with potential advantages for CTC survival and proliferation and to be responsible for micrometastatic lesions in distant organs (12). This finding was in accordance with the patient’s disease progression. As circulating endothelial cells can be present in patients with cancer and can be isolated by ISET creating a bias in CTCs counting, we performed a staining with anti-endothelial marker (CD34). No signal was detectable with the CD34 marker on the filters obtained from the patient while the positive and negative controls were satisfactory (data not shown). We thus concluded that the counting of CTCs obtained by ISET is accurate.
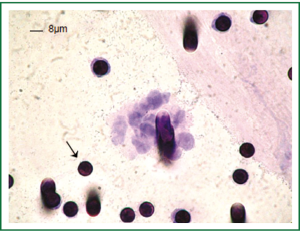
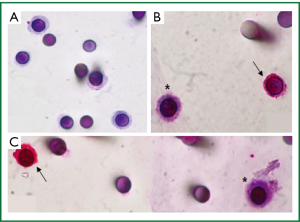
Figure 3A shows cells isolated by ISET, stained with hematoxylin-eosin on the membrane, whereas panels B and C show the distinction between CTCs and WBCs which were identified by a positive red CD45 staining. However, CTCs did not present immunopositivity for specific anti-CKs labelling, showing a different pattern of protein expression as compared with the patient primary tumor that was positive for CK7 and Pan CK (clone AE1/AE3). Furthermore, this result is in accordance with low number and heterogeneous CK+ CTCs found by the immunomagnetic Milteny’s methodology.
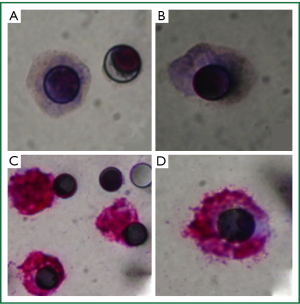
The pattern of CKs expression observed could be explained by a partial or incomplete EMT process, in which cells lose cell-to-cell adhesion and adopt some mesenchymal features, such as expression of vimentin and N-Cadherin, related to their increasing potential of invasion (13). For this reason, immunocytochemistry for N-Cadherin was performed and a weak staining was observed in the majority of CTCs isolated by ISET before the third cycle of chemotherapy (Figure 4A,B) pointing out the presence of a CTC population with mesenchymal features. We decided to look for other markers of EMT (E-cadherin and Vimentin) both in the first (before the first cycle of chemotherapy) and third (before the third cycle of chemotherapy) blood samples which were treated by ISET. E-cadherin expression was never found while vimentin expression was observed in double-staining with pan CK. Vimentin was highly expressed in the majority of CTCs from the first blood collection (Figure 4C,D), but there were some CTCs without any expression of this marker. Vimentin was moderately expressed in all CTCs isolated from the third blood collection. On the other hand, Pan CK expression, an epithelial marker, was negative in CTC isolated by ISET (first and third blood samples).
After three cycles of ineffective chemotherapy, the patient’s disease gradually progressed and she was initiated on palliative second-line chemotherapy with pemetrexed. This evolution was in agreement with CTC results (increased number and EMT) obtained by the antigen independent CTC isolation performed using ISET but not with CTC results (decreased number) obtained by the CK-based CTC isolation performed using Miltenyi.
Discussion
In the field of CTC, the issue of “CTC heterogeneity” is critical. It is well known that CTC may lose epithelial antigens and undergo EMT and that tumor cells in EMT are thought to have more “stem” characteristics including more invasive potential and self-renewal capacity (14). It is also known that tumor cells in EMT represent a relevant part of the CTC population in patients with lung cancer (7). Surprisingly, methods which only detect and count epithelial tumor cells and cannot identify CTC in EMT have been shown as having a prognostic value. In fact, Cell SearchTM analysis showed that 21% of evaluated 101 NSCLC patients were positive for CTCs (≥2 CTCs/7.5 mL blood, range, 0-149) and patients with 5 or more CTCs per 7.5 mL blood exhibited significantly worse overall survival (OS) compared to those patients with less than 5 CTCs (median OS, 4.3 versus 8.1 months respectively, P<0.001) (7). However, only 9 out of 101 patients, including 60 with metastases, had 5 or more CTCs per 7.5 mL and the mean survival time difference was of few months. Thus, practically, there is no at present clinical prognostic use of CTC enumeration, with an established cut-off level, for lung cancer patients.
In this setting, we planned to follow a single patient with metastatic lung cancer and compare CTC enumeration with both an “epithelial-antigen dependent” and an “epithelial-antigen independent” method to count CTC and analyzing the change in CTC number according to the clinical follow up. To our knowledge, this is the first study which has been designed to use clinical follow up and kinetic variation of the number of CTCs studied by two different approaches in order to obtain clues to identify the most accurate CTC method to be used in the follow up of patients with lung cancer.
Our results obtained using the “epithelial-antigen dependent” detection and counting of CTC showed a decrease in number of CTC during the disease course, suggesting a possible positive response to treatment. However, the patient’s clinical conditions worsened in paralleled. Of note is the fact that the last analysis by this technique showed CTC with different pattern of CK expression besides cells morphologically similar to CTC not expressing CK (Figure 1D).
In contrast, our results obtained using the “epithelial-antigen independent” ISET methodology, which was performed in parallel, showed an increasing number of CTC which was consistent with the observed worsening of clinical conditions and absence of response to treatment. According to previous reports using ISET we thought that these discrepancy could be related to CTC with EMT phenotype which are not detected by a technique based on CK expression, since progressive loss of epithelial and gain of mesenchymal markers are characteristics of the EMT process (14).
EMT has been associated with self-renewal and survival of CTCs with higher metastatic potential (15). Our results obtained with the ISET platform demonstrated a population of CTC with weak expression of N-Cadherin, a protein that contributes to EMT by stimulating invasive signaling in tumor cells (16). We also observed non-expression of E-cadherin in CTC collected by ISET at two different time-points. Qi et al. (17) showed N-Cadherin as a key protein in transendothelial migration of melanoma cells. In human prostate carcinoma, the overexpression of N-cadherin was associated with loss of E-cadherin and correlated with high Gleason score as well as with systemic and metastatic recurrence after surgery (18).
Hou et al. (12) demonstrated heterogeneous expression of N-Cadherin in CTC and CTM isolated by ISET platform from lung cancer patients which was described as a partial EMT phenotype. In our study, besides expression of N-Cadherin, another evidence for EMT was the fact that CTCs did not show CK7 or CK8 expression. This finding is in agreement with Krebs et al. (7) who found epithelial marker negative subpopulation of CTC isolated by ISET from chemo-naive, (stages IIIA to IV) NSCLC patients. Vimentin expression in CTC found in this study is in agreement with findings published by other authors (19), who also observed CTC expressing only vimentin or co-expressing it with keratin antigens at a very low or undetectable level in CTC from 6 patients with NSCLC. Bonnomet et al. (20) observed, using an experimental tumor model, that tumor cells in vascular tumoral emboli all expressed vimentin as also tumoral emboli in the lungs. However, macrometastases displayed heterogenous vimentin expression, as seen in the primary xenografts.
These findings strongly suggest that a relevant number of CTC in our patient were in EMT and detected by ISET but not detected by the method based on CK expression (Miltenyi).
In our study, the invasive potential of CTC was also shown by the presence of CTM detected by the ISET platform, but not by the immunomagnetic Miltenyi method. Presence of CTM in lung cancer patients was also mentioned by Hou et al. (12): ISET method showed heterogeneous expression of epithelial and mesenchymal markers in the evaluated CTM whereas Cell SearchTM technique found no evidence of CTM.
The relation between CTCs and tumor progression is supported by CTC dissemination and genetic mutations in CTC related to resistance to treatment (15). Maheswaran et al. (21) isolated CTCs from metastatic NSCLC patient carrying a resistance mutation to EGFR inhibitors, and demonstrated the presence of the mutation in pretreatment tumor-biopsy specimens which correlated with a reduced progression-free survival (7.7 vs. 16.5 months, P<0.001).
To summarize, in our case, the number of CTC detected by immunomagnetic labeling (Miltenyi) decreased and the number of CTC counted by ISET increased while the patient clinical conditions evolved to unfavorable prognosis. This may suggest that CTC in EMT were resistant to chemotherapy and continued to expand under therapy while the “epithelial” more differentiated CTC were sensitive to the therapy as their number decreased.
We concluded that the method which only identifies epithelial cells does not allow to assess the effect of the therapy and brings a CTC counting bias. At least for patients with lung cancer, counting CTC with a methodology only based on epithelial antigens and drawing conclusions on the response of patients to therapy seems unreliable.
In conclusion, by studying a single case of a patient with lung cancer and few months of survival after cancer diagnosis, we have obtained data suggesting that an unbiased enumeration of CTC taking into account their heterogeneity and variable expression of epithelial markers is more susceptible to be consistent with the clinical follow up. The method ISET for CTC detection allows to isolate CTCs independently from their expression of epithelial antigens. Our data are in agreement with previous reports (8) and suggest that ISET could provide relevant results when used for CTC counting in follow up of patients with lung cancer. More studies are now clearly needed providing validation of our data in larger clinical cohorts studies.
Acknowledgements
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Nadal R, Fernandez A, Sanchez-Rovira P, et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res 2012;14:R71. [PubMed]
- Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 2004;10:8152-62. [PubMed]
- Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012;106:508-16. [PubMed]
- Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2012;23:30-8. [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57-63. [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [PubMed]
- Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011;17:827-35. [PubMed]
- Ross JS, Slodkowska EA. Circulating and disseminated tumor cells in the management of breast cancer. Am J Clin Pathol 2009;132:237-45. [PubMed]
- Ignatiadis M, Reinholz M. Minimal residual disease and circulating tumor cells in breast cancer. Breast Cancer Res 2011;13:222. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [PubMed]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006;66:8319-26. [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [PubMed]
- Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer--clinical applications. Nat Rev Clin Oncol 2010;7:693-701. [PubMed]
- Agiostratidou G, Hulit J, Phillips GR, et al. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia 2007;12:127-33. [PubMed]
- Qi J, Chen N, Wang J, et al. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell 2005;16:4386-97. [PubMed]
- Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 2011;9:997-1007. [PubMed]
- Bonnomet A, Syne L, Brysse A, et al. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 2012;31:3741-53. [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [PubMed]


