Optimising drug dosing in patients receiving extracorporeal membrane oxygenation
Introduction
Temporary mechanical cardiorespiratory support with the use of extracorporeal membrane oxygenation (ECMO) circuitry in adult patients is a relatively new development in medicine (1-3). Technological advancements have allowed use of these therapies with relative ease and the patient outcomes are promising, even when it is applied as a rescue therapy (4). As ECMO finds its place in modern intensive care unit (ICU), clinical application of this technology needs further refinement to optimise its effectiveness. Currently, there is lack of clarity on many aspects of ECMO management and it may take decades of further research to generate evidence that will guide best practice. There is undoubtedly a learning curve for such complex interventions and clinical application of ECMO is rapidly evolving. For example, sedation on ECMO can be a significant challenge (5,6), but a patient being kept bed-bound whilst receiving ECMO and with heavy sedation is no longer a common practice in advanced ECMO centres and there is increasing emphasis on early interruption of sedation and use of mobilisation techniques (7-9). Optimal ventilation strategies on ECMO are also unclear and this has a significant bearing on sedation practices. Similarly, modern circuitry has allowed safe conduct of ECMO with low intensity anticoagulation (10). Very few centres now administer prophylactic antibiotics to their ECMO patients. Therefore, pharmacotherapy needs to be applied in a goal-directed fashion. Often, ECMO is a bridge to further long-term devices or transplant (11,12), and once again optimal pharmacotherapy helps clinicians to manage the ECMO run in a manner that minimises complications and maximises the likelihood of a patient receiving destination therapy. A thorough understanding of altered pharmacokinetics/pharmacodynamics (PK/PD) during ECMO is essential to apply pharmacotherapy in these complex patients (13), and this paper aims to provide the necessary background for clinicians and researchers in this field.
Pharmacotherapy to reverse disease
Mechanical support does not necessarily replace pharmacological support and optimal pharmacological management is critical to reverse underlying disease and minimise complications. Such drug therapy may include:
- Antibiotics or immunomodulator drugs that are administered to reverse underlying disease;
- Sedation and analgesia to minimise pain, discomfort and anxiety;
- Anticoagulation to minimise thrombotic risks within the patient and in the circuitry;
- Vasoactive drugs to support circulation or promote native cardiac ejection;
- Diuretic agents to assist fluid balance;
- Other general supportive measures.
It is important that these drugs are dosed effectively to achieve desired clinical effect which includes minimising the possibility of drug toxicity. While vasoactive drugs, sedatives, diuretics or anticoagulants can be titrated to real-time observable clinical endpoints, antibiotic dosing is often arbitrary or based on data from critically ill patients not on ECMO, or even from non-critically ill patients (14). This is concerning as reversal of the underlying disease that leads to cardiac and/or pulmonary dysfunction is critical to liberation from ECMO and not all patients may be bridged to other definitive options such as a long-term device or transplantation.
Therefore, the increasing use of ECMO in adult ICUs needs to be accompanied by an in-depth understanding on the potential ECMO-related PK changes and how these alterations may affect dosing requirements. The current challenge therefore is to investigate the ECMO-related PK changes in these drugs to in order to design optimised drug dosing regimens. Extreme PK changes in critically ill patients have been well described (14), and the introduction of ECMO appears to have added an additional level of complexity and importantly, another variable inadequately characterised in adult critical care management (15). Preliminary investigations have demonstrated that the introduction of ECMO potentially leads to significant changes in the PK of drugs in three ways: (I) drug sequestration by the circuit; (II) increased volume of distribution (Vd) and; (III) altered drug clearance (CL).
ECMO and drug sequestration
The interaction between the ECMO circuit and the physicochemical properties of drugs may lead to significant changes in the PK of many important drugs, subsequently altering the dosing requirements for patients on ECMO. An advanced understanding on this intricate interaction is critical to drug dosing in patients receiving ECMO, at least until more robust dosing guidelines become available.
Circuit factors
ECMO circuitry, which includes the conduit tubings and oxygenator membrane, introduces additional extracorporeal volume and increases the surface areas that drugs can be trapped in and adsorbed on. This results in an increase in Vd and subsequent decreases in plasma drug concentrations (15,16). However, the adsorption phenomenon may decrease over time due to saturation of binding sites and it is imperative that dosing of drugs also reflects this situation; applying higher dosing continuously during ECMO to overcome this adsorption phenomenon may later lead to drug toxicity. Conversely, the circuit may serve as a reservoir and redistributes the sequestered drug back into the patient even after the drug administration stops, potentially leading to prolonged undesirable pharmacological effects. Sequestration of drugs can be influenced by the following circuit factors: oxygenator materials (17-19); the types of conduit tubings (19,20); circuit age (20-22) and; the composition of the priming solution (23-25).
Physicochemical characteristics of the drug
The physicochemical properties of a compound determine the interaction of an individual drug with the ECMO circuit. The specific physicochemical characteristics that need to be considered include molecular size, pKa and degree of ionisation, lipophilicity and plasma protein binding (15). Numerous ex vivo data have suggested that the degree of lipophilicity and protein binding are significant factors affecting the proportion of drug sequestered within the ECMO circuit (23,24,26-28). The lipophilicity of a drug is generally described by the n-octanol/water partition coefficient (log P); a high positive log P indicates a higher degree of lipophilicity and therefore, drugs with such a property tend to be highly sequestered and extracted by the ECMO circuit than drugs with a lower log P (18,28,29). Additionally, the extent of protein binding may determine the degree of drug sequestration for drugs with similar lipophilicity (26,29).
ECMO and increased volume of distribution
The introduction of ECMO may alter the apparent Vd of drugs by the following mechanisms: (I) drug sequestration; (II) haemodilution from priming solution and; (III) ECMO-related physiological changes. PK changes associated with the systemic inflammatory response syndrome (SIRS) are common in critically ill patients receiving ECMO, potentially leading to an increase in Vd of hydrophilic drugs (30). Furthermore, patients receiving ECMO may have significant changes in blood pH leading to further alterations in drug distribution, degree of ionisation and protein binding (31). Numerous data documenting the relationship between ECMO usage and enlarged Vd mostly originated from ex vivo and neonatal PK studies (16,32,33). Hence, the extrapolation of this data to critically ill adult patients may potentially be misleading due to significant physiological and body composition differences between these populations and must be undertaken with caution.
ECMO and drug clearance
In general, ECMO patients have been shown to display lower drug CL when compared with patients not receiving ECMO (16). Lower drug CL and the resultant accumulation of drugs and their metabolites are believed to be caused by renal and hepatic hypoperfusion and hypoxia (34). This phenomenon is sometimes offset by the initial increase of clearance due to increased cardiac output secondary to SIRS, aggressive fluid therapy and inotropic support (30).
Dosing regimens based on the CL of non-ECMO population are often used to guide dosing in ECMO patients due to scarcity of more relevant data. As previously discussed, the PK parameters in this subpopulation are likely to be unique. Much of the current ECMO PK data exist in the neonatal literature (35), but extrapolation from this must be done with caution in the context of the immature glomerular and tubular function, as well as the developing hepatic function of newborns (36).
The impact of ECMO on the PK of antimicrobials
Much of the emphasis and incentive to better understand ECMO-related PK changes stems from the desire to provide optimal antibiotic therapy for critically ill ECMO patients. Optimal antibiotic therapy has been shown to correlate with improved patient outcomes (37-39) and this strategy often underpins the primary treatment goal in this patient population. In comparison to sedatives and vasoactive agents, which can be titrated to effect, antibiotic therapy is guided by laboratory surrogates without any real-time feedback. Suboptimal antibiotic dosing may likely lead to treatment failures and importantly, to the development of bacterial resistance (40). Optimal dosing of antibiotics must consider physicochemical characteristics of the individual drug and its interaction with the circuit (26,28,29). In the absence of robust data guiding antibiotic dosage for patients on ECMO, it is prudent that the use of existing published data is supplemented with therapeutic drug monitoring (TDM) where possible. Table 1 summarizes the potential PK changes and suggested dosing adjustments for several important antimicrobials during ECMO support.
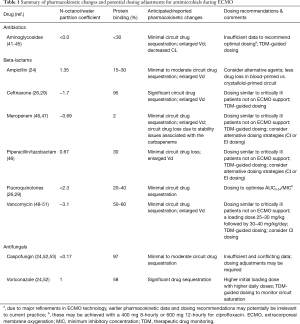
Full table
Beta-lactams
Beta-lactam antibiotics are relatively hydrophilic with varying levels of protein binding. This suggests that there may be inter-class variability in their ECMO-related PK changes. For example, ceftriaxone is ≥85% protein-bound and would likely have higher drug sequestration into the ECMO circuitry than other beta-lactams with lower protein-binding properties (26,29). The beta-lactam’s optimal bactericidal killing is achieved when the free (unbound) drug concentration remains four-to-five times above the minimum inhibitory concentration (MIC) of a pathogen for extended periods during a dosing interval (fT>MIC) (54,55). Existing dosing regimens aim to optimise this index but with the ECMO- and critical illness-related PK changes, the ability to ensure optimal fT>MIC becomes a clinical challenge.
Numerous ex vivo model of ECMO circuits (26,28,56,57) and clinical PK studies (46,47,58,59) have investigated beta-lactams PK during ECMO support. The earlier studies demonstrated high variability in drug concentrations with a largely unpredictable PK profile when comparing ECMO versus non-ECMO patients. However, two recent studies have both suggested that the introduction of the ECMO does not significantly alter the PK of beta-lactams (46,47). As the presence of ECMO has not been found to significantly alter the PK of beta-lactam antibiotics, the recommended dosing strategies for critically ill patients without ECMO support can be applied in this patient population (60). It is also suggested to use beta-lactam TDM during ECMO to maximise therapeutic outcomes (60,61).
Vancomycin
This glycopeptide antibiotic exhibits optimal bacteriological and clinical outcomes when the ratio of area under the concentration-time curve during a 24-hour period (AUC0–24) to MIC (AUC0–24/MIC) is maintained ≥400 (62,63). Early adult and neonatal PK studies suggested that there is an ECMO-related increase in Vd and decrease in CL and as a result alterations in effective vancomycin concentrations (64-66). Many newer studies have not corroborated these earlier findings (48-51). A retrospective PK study showed that conventional intermittent vancomycin dosing may likely be a flawed dosing strategy during ECMO, with 95% of the cohort achieving suboptimal antibiotic exposure (50). However, a matched-cohort study conducted by Donadello et al. showed similar Vd and CL between ECMO and non-ECMO patients, who all received continuous vancomycin infusion (51). This suggests that the use of continuous vancomycin infusion during ECMO may potentially negate the ECMO-related PK changes. Higher vancomycin doses may be required if intermittent bolus dosing is used in patients receiving ECMO.
Fluoroquinolones
Similar to vancomycin, optimal bactericidal killing of fluoroquinolones is associated with the AUC0–24/MIC ratio (67). There are limited PK data available on this class of antibiotic in ECMO patients, but an ex vivo experiment has suggested that the risk of circuit drug loss is relatively low and potentially insignificant for ciprofloxacin (26). Given other members of the group such as moxifloxacin and levofloxacin have a lower log P values with similar protein binding properties as compared to ciprofloxacin (68), it is therefore anticipated that the degree of sequestration in ECMO circuit for these drugs may also be relatively low. It is highly likely that no dosing adjustment is required when these antibiotics are used during ECMO but dosing should seek to maximise AUC0–24/MIC ratio as this ensures optimal therapeutic outcomes in critically ill patients (67). Nevertheless, more robust PK data are urgently needed to corroborate these preliminary data.
Aminoglycosides
Limited data has been published on the PK of aminoglycosides in the critically ill adult population receiving ECMO. Aminoglycoside exhibits concentration-dependent bacterial killing and optimal therapeutic outcomes have been associated with achieving a ratio of peak drug concentration (Cmax) to MIC (Cmax/MIC) ratio of 10–12 (69) and an AUC0–24/MIC ratio of 80–160 (70). Neonatal PK studies have mostly reported an increase in the Vd of gentamicin with a decrease in CL (41-45). However, extreme caution must be taken when extrapolating these findings to the critically ill adult patients given marked differences in patient physiology and rapid evolution of ECMO technology, rendering earlier PK knowledge and dosing recommendations irrelevant to current clinical practice. It is also recommended that TDM should continue to be employed when an aminoglycoside is used in patients receiving ECMO.
Antifungals
Antifungals are heterogeneous group of drugs with varying levels of protein binding and lipophilicity. With limited large-scale PK data, their optimal dosing in ECMO remains arbitrary. Voriconazole demonstrated significant sequestration into the ECMO circuit in an ex vivo study (24), with 71% of circuit drug loss being reported, which was expected due to the drug’s high log P value and moderate protein binding property (68). Another published case study of voriconazole in ECMO patients corroborated the ex vivo finding, highlighting the need for an increased voriconazole dosing for this subpopulation (52). However, Spriet et al. also demonstrated time-dependent saturation of the circuit leading to supra-therapeutic concentrations with increased dosing.
The echinocandin antifungal, caspofungin, has also been studied in ex vivo model of ECMO circuit reporting a 43% loss due to sequestration (24). However, several reports in critically ill adult patients documented conflicting findings (52,53). Further, larger powered studies are required to investigate the PK of antifungals in patients on ECMO support.
The impact of ECMO on the PK of sedatives and analgesics
Significant amounts of research have been dedicated towards optimising sedation and analgesia for critically ill patients. Evidence-based sedation protocols, primarily aiming for lighter and minimal sedation, have been suggested to improve therapeutic outcomes in critically ill patient population (9). However, strict adherence to these recommendations may be challenging in critically ill patients receiving ECMO and importantly, may not always be practical in such a scenario. Earlier in the course of extracorporeal support, patients on ECMO usually require deep sedation and paralysis to optimise circuit flows and ventilation whilst eliminating pain, anxiety and other forms of distress induced by the ICU environment (71). Further to this, emerging reports have documented greater sedative requirements are needed for critically ill neonates (33,59,72,73) and adults during ECMO support (5,6). PK studies in neonates have consistently reported increased Vd and decreased drug CL during ECMO (16,35), both of which could potentially explain the heightened sedation requirement observed in this patient cohort. Table 2 summarizes the potential PK changes and suggested dosing adjustments for several important sedatives and analgesics during ECMO support.
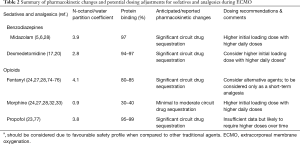
Full table
Opioids
Fentanyl, due to its higher degree of lipophilicity (68), appears to be more significantly sequestered in the ECMO circuit as opposed to other drugs. In a recent ex vivo experiment, Shekar et al. demonstrated that the mean drug loss of fentanyl (97%) to ECMO circuits was relatively higher when compared to those of morphine (0%) and midazolam (87%) (28). This, among other studies (24,27,74-76), corroborates the need to escalate fentanyl doses over time in order to achieve optimal sedation in critically ill patients receiving ECMO (5,6). However, higher fentanyl doses for this cohort may not always be feasible and safe; excessive sedative drug use has been associated with increased patient morbidity in the ICU (78-81). Fentanyl therefore appears to be an inferior option in this population and if it is to be used, it may be considered as a short-term alternative to other agents. In an ex vivo model of ECMO circuit, Mehta et al. observed that fentanyl concentrations remained stable for up to 3 hours but were undetectable at 24 hours during their experiment (24).
In contrast, the impact of ECMO on morphine PK is less pronounced when compared to fentanyl (24,27,28,32,33). Shekar et al. demonstrated that the average morphine recovery from their ex vivo model of ECMO circuits at 24 hours was >99% and this suggests that the risk of drug sequestration to the circuit is relatively low and potentially insignificant for morphine (28). This, among other findings (18,24,27,32,33), may likely stem from the result of morphine being less lipophilic (68), and consequently, making it a superior clinical option as opposed to fentanyl in the management of ECMO patients. Additionally, morphine has also been reported to provide optimal analgesia, whilst reducing drug withdrawal and length of hospital stay significantly.
Although most of the available data noted greater opioid requirements on ECMO, a more recent publication indicated otherwise. In a 2-year, prospective, observational study involving 32 critically ill patients on ECMO, DeGrado et al. observed that this cohort required relatively lower doses of opioids than previously described with no dose increments needed throughout the study duration (82). It is imperative to highlight that ≥20% of the cohort were receiving ECMO as a bridge to transplantation and this subgroup of patients commonly requires lower sedative and analgesic doses compared with other indications for ECMO (83). Furthermore, sedation practices vary across different institutions and may contribute to these contradictory findings. DeGrado et al. also noted that the patients who received venovenous (VV) ECMO in this study also had significantly higher opioid requirements than those receiving venoarterial (VA) ECMO (82). Approximately half of the VV ECMO patients had acute respiratory distress syndrome (ARDS) and therefore were more likely to need higher doses of sedatives and analgesics, before and during ECMO treatment, to facilitate optimal mechanical ventilation. In contrast, VA ECMO patients may have been fully anaesthetised prior to or during cardiac surgery.
Benzodiazepines
Significant midazolam sequestration has been observed in several neonatal and paediatric ECMO studies (59,84). In an ex vivo model of ECMO circuit, Shekar et al. aimed to describe midazolam disposition in the adult ECMO circuitry (28). In this experiment, the average midazolam recovery from the ECMO circuits was approximately 13% and more importantly, half of the drug was lost in the circuits within 1 hour of the experiment. This essentially means that higher initial midazolam doses may be required to achieve early and optimal sedation in critically ill patients during ECMO support. These findings are further corroborated by two retrospective cohort studies (5,6), which aimed to characterise sedation requirements in patients receiving ECMO for cardiac and/or respiratory failure. In the first study, Shekar et al. reported that the median daily dose for midazolam was 175 mg (range, 24–1,500 mg), representing a 10% increase in daily dose after ECMO commencement (6). Furthermore, these patients were receiving up to 1,500 mg of midazolam/day despite concomitant use of propofol, dexmedetomidine, thiopentone and neuroleptic agents, raising genuine concerns of increased morbidity secondary to excessive sedation. In the second study, Nigoghossian et al. documented that ECMO patients required twice as high midazolam 6-hour exposure when compared to patients not on ECMO support (ECMO: 118 mg vs. non-ECMO: 60 mg ; P=0.04) and optimal sedation was reached approximately three days later in the ECMO group (5).
However, these earlier findings have since been contradicted by a recent publication and therefore warrant further investigation. In a cohort of 32 critically ill patients receiving ECMO, DeGrado et al. recently noted that these patients required significantly lower doses of benzodiazepines (24 mg) than previously documented in the literature and additionally, did not demonstrate a need for dose escalation throughout ECMO treatment (82). When patients who received ECMO as a bridge to transplantation were excluded from analysis, benzodiazepine requirements were significantly higher in the VV ECMO group (VV ECMO: 48 mg vs. VA ECMO: 34 mg; P=0.006), similar to what has been reported by Shekar et al. (6).
Dexmedetomidine
Dexmedetomidine is unique compared with other traditional agents, because it produces sedation and analgesia without compromising respiratory drive (85,86). However, limited data currently exist on its usage in critically ill patients during ECMO support (17,20,87). Due to the lipophilic nature of the drug, dexmedetomidine appears to be significantly lost through circuit adsorption during ECMO (68). Wagner et al. used an in vitro model to evaluate the impact of ECMO on the disposition of dexmedetomidine over the course of 24 hours (20). Within the first hour of the experiment, ≥40% of the drugs were lost in the circuits and only ≤30% remained at 24 hours. The investigators further suggested that the significant drug loss was likely due to adsorption to polyvinyl chloride (PVC) tubings and this notion has also been supported by a recent publication (17). The precise role of dexmedetomidine remains unclear in patients receiving ECMO but its favourable safety profile may be advantageous over other traditional agents. On the basis of the available data, using higher initial loading doses with higher daily doses may be considered to compensate for the anticipated PK changes during ECMO treatment.
Propofol
As propofol is a highly lipophilic and highly protein bound drug (68), it is anticipated to be significantly sequestered within the ECMO circuitry (21,77,88-90). In an in vitro study, Hynynen et al. showed that approximately 35% and 75% of propofol were lost within the ECMO circuits after 5 and 120 minutes of the experiment, respectively (89). In a recent ex vivo model of whole-blood primed ECMO circuit, Lemaitre et al. reinforced earlier experimental findings which documented significant propofol loss through circuit adsorption (23). In this study, 70% of drug concentration diminished within the first 30 minutes of the experiment and after 5 hours, only 11% of the initial propofol concentration remained. Although these findings are expected based on the physicochemical properties of propofol, Lemaitre et al. also suggested that oxidation may also be an important determinant of propofol PK during ECMO; a 70% decrease in propofol concentrations were noted at 45 minutes post-oxygen exposure in the in vitro arm of the study. Based on these limited data, it appears that higher doses of propofol may be required over time for optimal sedation but further studies are needed to investigate the safety of such an approach in ECMO patients.
Anecdotally, in the author’s experience, a multimodal strategy of early tracheostomy/extubation where feasible, a combination of enteral longer-acting benzodiazepines and antipsychotics, intravenous short acting benzodiazepine and an opioid (morphine preferred) and dexmedetomidine in titrated doses to clinical endpoints often achieves optimal sedation, analgesia and anxiolysis. Intravenous ketamine can be a useful adjunct. Enteral methadone may be added as the risks of opioid withdrawal after weeks of high dose opioid therapy may be substantial and often excessive sedation is administered to overcome this. Dexmedetomidine, being a negative chronotropic agent can also aid optimisation of oxygenation in VV ECMO patients who have refractory hypoxia on ECMO in the setting of a high native cardiac output. Development of evidence-based sedation and analgesia targets and protocols for ECMO patients may help address this complex issue moving forward.
The impact of ECMO on the PK of other drugs
There are limited data available on the PK of other drugs during ECMO and most of them originated from neonatal and paediatric studies. Scarce PK data are available for several cardiovascular drugs (91-95), diuretics (96-98), phenobarbital (99), ranitidine (100), and theophylline (101), and these studies generally reported altered drug PK and dosing requirements during ECMO support.
Anticoagulants
Optimising anticoagulation in patients receiving ECMO is necessary to prevent some of the common ECMO-related complications, such as bleeding and/or thrombosis. The PK of heparin was studied on five infants receiving ECMO and in this study, Green et al. found that more than one-half of the administered heparin was cleared by the circuit itself or by blood components in the circuit (102). Data from an ex vivo ECMO model further corroborate this observation whereby approximately 30% and 50% of heparin were lost at 24 hours in the crystalloid- and blood-primed ECMO circuits, respectively (24). A more recent study by Park et al. however appears to contradict these earlier findings and their in vitro ECMO model essentially highlights the importance of material selection on ECMO-related PK changes (17). In this study, the authors found that heparin concentrations remain unchanged in the different types of tubings and oxygenators tested.
Future directions
In order to optimise drug dosing in critically ill patients receiving ECMO, it is essential that the relative impact of drug, device and critical illness factors are considered and systematically investigated, either in isolation or combination. This can be achieved by integrating pre-clinical findings (e.g., ex vivo and animal ECMO models) with data from critically ill ECMO patients and through these processes, the PK of a drug and sources for its variability can be described and importantly, optimal dosing recommendations could be suggested for this patient population. In this respect, the ECMO PK Project (103) and the ASAP ECMO Study (104) are two prime examples of such an approach, combining mechanistic and clinical research to investigate PK alterations during ECMO. Data from the two studies will identify the drugs that are most suitable to be used during ECMO and strategies to optimise dosing in critically ill patients receiving ECMO in accordance to PK/PD principles. Until robust dosing guidelines become available, physicochemical properties of drugs can be used to predict PK changes and consequently, guide effective dosing in this patient population.
Conclusions
Optimised pharmacotherapy enhances the effectiveness of ECMO and is crucial to its success. Clinicians should use available therapeutic drugs in a manner that allows the best possible clinical application of ECMO. Further refinements in clinical application of ECMO will provide clear PD endpoints for many of the drugs that are commonly used on ECMO. In the meantime, pending population PK data and dosing guidelines, a sound understanding of altered PK in critically ill and those on ECMO will serve a guide to drug dosing on ECMO.
Acknowledgements
Funding: The authors wish to recognise funding from the Australian National Health and Medical Research Council for Centres of Research Excellence (CRE REDUCE APP1099452 and CRE ACTIONS APP1079421) and a Practitioner Fellowship to JAR (APP1117065). V Cheng acknowledges funding for a PhD scholarship from both CRE REDUCE and CRE ACTIONS and MHAA a post-doctoral Fellowship from CRE REDUCE. The authors also wish to acknowledge the funding from the research centres they are affiliated to.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shekar K, Gregory SD, Fraser JF. Mechanical circulatory support in the new era: an overview. Crit Care 2016;20:66. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Shekar K. Extracorporeal respiratory support: breaking conventions? Anaesth Intensive Care 2014;42:175-7. [PubMed]
- Shekar K, Mullany DV, Thomson B, et al. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 2014;18:219. [Crossref] [PubMed]
- Nigoghossian CD, Dzierba AL, Etheridge J, et al. Effect of Extracorporeal Membrane Oxygenation Use on Sedative Requirements in Patients with Severe Acute Respiratory Distress Syndrome. Pharmacotherapy 2016;36:607-16. [Crossref] [PubMed]
- Shekar K, Roberts JA, Mullany DV, et al. Increased sedation requirements in patients receiving extracorporeal membrane oxygenation for respiratory and cardiorespiratory failure. Anaesth Intensive Care 2012;40:648-55. [PubMed]
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014;18:R38. [Crossref] [PubMed]
- Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263-306. [Crossref] [PubMed]
- Kress JP, Vinayak AG, Levitt J, et al. Daily sedative interruption in mechanically ventilated patients at risk for coronary artery disease. Crit Care Med 2007;35:365-71. [Crossref] [PubMed]
- Weingart C, Lubnow M, Philipp A, et al. Comparison of Coagulation Parameters, Anticoagulation, and Need for Transfusion in Patients on Interventional Lung Assist or Veno-Venous Extracorporeal Membrane Oxygenation. Artif Organs 2015;39:765-73. [Crossref] [PubMed]
- Strueber M. Bridges to lung transplantation. Curr Opin Organ Transplant 2011;16:458-61. [Crossref] [PubMed]
- Thiagarajan RR, Brogan TV, Scheurer MA, et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg 2009;87:778-85. [Crossref] [PubMed]
- Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit Care 2017;21:66. [Crossref] [PubMed]
- Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014;14:498-509. [Crossref] [PubMed]
- Ha MA, Sieg AC. Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation. Pharmacotherapy 2017;37:221-35. [Crossref] [PubMed]
- Shekar K, Fraser JF, Smith MT, et al. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 2012;27:741.e9-18. [Crossref] [PubMed]
- Park J, Shin DA, Lee S, et al. Investigation of Key Circuit Constituents Affecting Drug Sequestration During Extracorporeal Membrane Oxygenation Treatment. Asaio j 2017;63:293-8. [Crossref] [PubMed]
- Wildschut ED, Ahsman MJ, Allegaert K, et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med 2010;36:2109-16. [Crossref] [PubMed]
- Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion 2005;20:309-15. [Crossref] [PubMed]
- Wagner D, Pasko D, Phillips K, et al. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion 2013;28:40-6. [Crossref] [PubMed]
- Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion 2000;15:21-6. [Crossref] [PubMed]
- Dagan O, Klein J, Gruenwald C, et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit 1993;15:263-6. [Crossref] [PubMed]
- Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care 2015;19:40. [Crossref] [PubMed]
- Mehta NM, Halwick DR, Dodson BL, et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 2007;33:1018-24. [Crossref] [PubMed]
- Mulla HG, Firmin RK, David RU. Drug disposition during extracorporeal membrane oxygenation (ECMO). Paediatr Perinat Drug Ther 2001;4:109-20.
- Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 2015;19:164. [Crossref] [PubMed]
- Harthan AA, Buckley KW, Heger ML, et al. Medication adsorption into contemporary extracorporeal membrane oxygenator circuits. J Pediatr Pharmacol Ther 2014;19:288-95. [PubMed]
- Shekar K, Roberts JA, McDonald CI, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 2012;16:R194. [Crossref] [PubMed]
- Shekar K, Roberts JA, Barnett AG, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care 2015;19:437. [Crossref] [PubMed]
- Tsai D, Lipman J, Roberts JA. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care 2015;21:412-20. [Crossref] [PubMed]
- Bartlett RH. Extracorporeal life support for cardiopulmonary failure. Curr Probl Surg 1990;27:621-705. [Crossref] [PubMed]
- Geiduschek JM, Lynn AM, Bratton SL, et al. Morphine pharmacokinetics during continuous infusion of morphine sulfate for infants receiving extracorporeal membrane oxygenation. Crit Care Med 1997;25:360-4. [Crossref] [PubMed]
- Dagan O, Klein J, Bohn D, et al. Effects of extracorporeal membrane oxygenation on morphine pharmacokinetics in infants. Crit Care Med 1994;22:1099-101. [Crossref] [PubMed]
- Ulldemolins M, Roberts JA, Lipman J, et al. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011;139:1210-20. [Crossref] [PubMed]
- Himebauch AS, Kilbaugh TJ, Zuppa AF. Pharmacotherapy during pediatric extracorporeal membrane oxygenation: a review. Expert Opin Drug Metab Toxicol 2016;12:1133-42. [Crossref] [PubMed]
- Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev 2003;55:667-86. [Crossref] [PubMed]
- Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 2010;38:1773-85. [Crossref] [PubMed]
- Lodise TP Jr, Patel N, Kwa A, et al. Predictors of 30-day mortality among patients with Pseudomonas aeruginosa bloodstream infections: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 2007;51:3510-5. [Crossref] [PubMed]
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. [Crossref] [PubMed]
- Abdul-Aziz MH, Lipman J, Mouton JW, et al. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med 2015;36:136-53. [Crossref] [PubMed]
- Dodge WF, Jelliffe RW, Zwischenberger JB, et al. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther Drug Monit 1994;16:552-9. [Crossref] [PubMed]
- Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy 1992;12:28-32. [PubMed]
- Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans 1991;37:16-8. [Crossref] [PubMed]
- Cohen P, Collart L, Prober CG, et al. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J 1990;9:562-6. [Crossref] [PubMed]
- Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother 1989;33:817-9. [Crossref] [PubMed]
- Donadello K, Antonucci E, Cristallini S, et al. beta-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int J Antimicrob Agents 2015;45:278-82. [Crossref] [PubMed]
- Shekar K, Fraser JF, Taccone FS, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care 2014;18:565. [Crossref] [PubMed]
- Moore JN, Healy JR, Thoma BN, et al. A Population Pharmacokinetic Model for Vancomycin in Adult Patients Receiving Extracorporeal Membrane Oxygenation Therapy. CPT Pharmacometrics Syst Pharmacol 2016;5:495-502. [Crossref] [PubMed]
- Wu CC, Shen LJ, Hsu LF, et al. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc 2016;115:560-70. [Crossref] [PubMed]
- Park SJ, Yang JH, Park HJ, et al. Trough Concentrations of Vancomycin in Patients Undergoing Extracorporeal Membrane Oxygenation. PLoS One 2015;10:e0141016. [Crossref] [PubMed]
- Donadello K, Roberts JA, Cristallini S, et al. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care 2014;18:632. [Crossref] [PubMed]
- Spriet I, Annaert P, Meersseman P, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother 2009;63:767-70. [Crossref] [PubMed]
- Ruiz S, Papy E, Da Silva D, et al. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med 2009;35:183-4. [Crossref] [PubMed]
- Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003;17:479-501. [Crossref] [PubMed]
- Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998;26:1-10. [Crossref] [PubMed]
- Leven C, Fillatre P, Petitcollin A, et al. Ex Vivo Model to Decipher the Impact of Extracorporeal Membrane Oxygenation on Beta-lactam Degradation Kinetics. Ther Drug Monit 2017;39:180-4. [Crossref] [PubMed]
- Tron C, Leven C, Fillatre P, et al. Should we fear tubing adsorption of antibacterial drugs in extracorporeal membrane oxygenation? An answer for cephalosporins and carbapenems. Clin Exp Pharmacol Physiol 2016;43:281-3. [Crossref] [PubMed]
- Welsch C, Augustin P, Allyn J, et al. Alveolar and serum concentrations of imipenem in two lung transplant recipients supported with extracorporeal membrane oxygenation. Transpl Infect Dis 2015;17:103-5. [Crossref] [PubMed]
- Ahsman MJ, Wildschut ED, Tibboel D, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother 2010;54:1734-41. [Crossref] [PubMed]
- Roberts JA, Kumar A, Lipman J. Right Dose, Right Now: Customized Drug Dosing in the Critically Ill. Crit Care Med 2017;45:331-6. [Crossref] [PubMed]
- Jager NG, van Hest RM, Lipman J, et al. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 2016;9:961-79. [Crossref] [PubMed]
- Zelenitsky S, Rubinstein E, Ariano R, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents 2013;41:255-60. [Crossref] [PubMed]
- Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004;43:925-42. [Crossref] [PubMed]
- Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol 2005;60:265-75. [Crossref] [PubMed]
- Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother 1996;40:1139-42. [PubMed]
- Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm 1990;9:711-5. [PubMed]
- Zelenitsky SA, Ariano RE. Support for higher ciprofloxacin AUC 24/MIC targets in treating Enterobacteriaceae bloodstream infection. J Antimicrob Chemother 2010;65:1725-32. [Crossref] [PubMed]
- Wishart DS, Knox C, Guo AC, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 2006;34:D668-72. [Crossref] [PubMed]
- Kashuba AD, Nafziger AN, Drusano GL, et al. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother 1999;43:623-9. [PubMed]
- Drusano GL, Ambrose PG, Bhavnani SM, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 2007;45:753-60. [Crossref] [PubMed]
- Sessler CN, Wilhelm W. Analgesia and sedation in the intensive care unit: an overview of the issues. Crit Care 2008;12 Suppl 3:S1. [Crossref] [PubMed]
- Leuschen MP, Willett LD, Hoie EB, et al. Plasma fentanyl levels in infants undergoing extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 1993;105:885-91. [PubMed]
- Arnold JH, Truog RD, Scavone JM, et al. Changes in the pharmacodynamic response to fentanyl in neonates during continuous infusion. J Pediatr 1991;119:639-43. [Crossref] [PubMed]
- Hynynen M. Binding of fentanyl and alfentanil to the extracorporeal circuit. Acta Anaesthesiol Scand 1987;31:706-10. [Crossref] [PubMed]
- Skacel M, Knott C, Reynolds F, et al. Extracorporeal circuit sequestration of fentanyl and alfentanil. Br J Anaesth 1986;58:947-9. [Crossref] [PubMed]
- Koren G, Crean P, Klein J, et al. Sequestration of fentanyl by the cardiopulmonary bypass (CPBP). Eur J Clin Pharmacol 1984;27:51-6. [Crossref] [PubMed]
- Hammarén E, Rosenberg PH, Hynynen M. Coating of extracorporeal circuit with heparin does not prevent sequestration of propofol in vitro. Br J Anaesth 1999;82:38-40. [Crossref] [PubMed]
- Jackson DL, Proudfoot CW, Cann KF, et al. A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care 2010;14:R59. [Crossref] [PubMed]
- Loepke AW. Developmental neurotoxicity of sedatives and anesthetics: a concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med 2010;11:217-26. [Crossref] [PubMed]
- Ista E, van Dijk M, Gamel C, et al. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Crit Care Med 2008;36:2427-32. [Crossref] [PubMed]
- Ducharme C, Carnevale FA, Clermont MS, et al. A prospective study of adverse reactions to the weaning of opioids and benzodiazepines among critically ill children. Intensive Crit Care Nurs 2005;21:179-86. [Crossref] [PubMed]
- DeGrado JR, Hohlfelder B, Ritchie BM, et al. Evaluation of sedatives, analgesics, and neuromuscular blocking agents in adults receiving extracorporeal membrane oxygenation. J Crit Care 2017;37:1-6. [Crossref] [PubMed]
- Lehr CJ, Zaas DW, Cheifetz IM, et al. Ambulatory extracorporeal membrane oxygenation as a bridge to lung transplantation: walking while waiting. Chest 2015;147:1213-8. [Crossref] [PubMed]
- Mulla H, Lawson G, Peek GJ, et al. Plasma concentrations of midazolam in neonates receiving extracorporeal membrane oxygenation. Asaio j 2003;49:41-7. [Crossref] [PubMed]
- Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13-21. [Crossref] [PubMed]
- Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000;59:263-8; discussion 9-70. [Crossref] [PubMed]
- Anton-Martin P, Modem V, Taylor D, et al. A retrospective study of sedation and analgesic requirements of pediatric patients on extracorporeal membrane oxygenation (ECMO) from a single-center experience. Perfusion 2017;32:183-91. [Crossref] [PubMed]
- Myers GJ, Voorhees C, Eke B, et al. The effect of Diprivan (propofol) on phosphorylcholine surfaces during cardiopulmonary bypass--an in vitro investigation. Perfusion 2009;24:349-55. [Crossref] [PubMed]
- Hynynen M, Hammaren E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth 1994;41:583-8. [Crossref] [PubMed]
- Tarr TJ, Kent AP. Sequestration of propofol in an extracorporeal circuit. J Cardiothorac Anesth 1989;3:75. [Crossref] [PubMed]
- Ahsman MJ, Witjes BC, Wildschut ED, et al. Sildenafil exposure in neonates with pulmonary hypertension after administration via a nasogastric tube. Arch Dis Child Fetal Neonatal Ed 2010;95:F109-14. [Crossref] [PubMed]
- Kendrick JG, Macready JJ, Kissoon N. Amiodarone treatment of junctional ectopic tachycardia in a neonate receiving extracorporeal membrane oxygenation. Ann Pharmacother 2006;40:1872-5. [Crossref] [PubMed]
- Smith T, Rosen DA, Russo P, et al. Nesiritide during extracorporeal membrane oxygenation. Paediatr Anaesth 2005;15:152-7. [Crossref] [PubMed]
- McBride BF, White CM, Campbell M, et al. Nicardipine to control neonatal hypertension during extracorporeal membrane oxygen support. Ann Pharmacother 2003;37:667-70. [Crossref] [PubMed]
- Tobias JD, Pietsch JB, Lynch A. Nicardipine to control mean arterial pressure during extracorporeal membrane oxygenation. Paediatr Anaesth 1996;6:57-60. [Crossref] [PubMed]
- van der Vorst MM, den Hartigh J, Wildschut E, et al. An exploratory study with an adaptive continuous intravenous furosemide regimen in neonates treated with extracorporeal membrane oxygenation. Crit Care 2007;11:R111. [Crossref] [PubMed]
- van der Vorst MM, Wildschut E, Houmes RJ, et al. Evaluation of furosemide regimens in neonates treated with extracorporeal membrane oxygenation. Crit Care 2006;10:R168. [Crossref] [PubMed]
- Wells TG, Fasules JW, Taylor BJ, et al. Pharmacokinetics and pharmacodynamics of bumetanide in neonates treated with extracorporeal membrane oxygenation. J Pediatr 1992;121:974-80. [Crossref] [PubMed]
- Elliott ES, Buck ML. Phenobarbital dosing and pharmacokinetics in a neonate receiving extracorporeal membrane oxygenation. Ann Pharmacother 1999;33:419-22. [Crossref] [PubMed]
- Wells TG, Heulitt MJ, Taylor BJ, et al. Pharmacokinetics and pharmacodynamics of ranitidine in neonates treated with extracorporeal membrane oxygenation. J Clin Pharmacol 1998;38:402-7. [Crossref] [PubMed]
- Mulla H, Nabi F, Nichani S, et al. Population pharmacokinetics of theophylline during paediatric extracorporeal membrane oxygenation. Br J Clin Pharmacol 2003;55:23-31. [Crossref] [PubMed]
- Green TP, Isham-Schopf B, Irmiter RJ, et al. Inactivation of heparin during extracorporeal circulation in infants. Clin Pharmacol Ther 1990;48:148-54. [Crossref] [PubMed]
- Shekar K, Roberts JA, Smith MT, et al. The ECMO PK Project: an incremental research approach to advance understanding of the pharmacokinetic alterations and improve patient outcomes during extracorporeal membrane oxygenation. BMC Anesthesiol 2013;13:7. [Crossref] [PubMed]
- Shekar K, Roberts JA, Welch S, et al. ASAP ECMO: Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: a multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol 2012;12:29. [Crossref] [PubMed]


