Clinical analyses on salvage lymphadenectomy through cervical incision for patients with cervical and cervicothoracic recurrences after esophagectomy
Introduction
Lymph nodes are often involved across the regions due to the special anatomic characteristics (1). Despite the efforts of extended lymphadenectomy, locoregional recurrences are often observed (2,3). The strategy of salvage therapy for patients with locoregional recurrences has not been established completely (4-6). In our center, for patients with cervical and cervicothoracic recurrences, salvage lymphadenectomy though cervical incision has been performed for years (7-9). We reviewed all patients underwent initial esophagectomy and secondary salvage lymphadenectomy in our center during July 2006 and September 2016. The study aimed to describe results of salvage lymphadenectomy and determine prognostic factors after the procedure.
Methods
Patients
During the period between July 2006 and September 2016, there were 75 cases underwent salvage lymphadenectomy through cervical incision for cervical and cervicothoracic recurrences. In this study, the recurrence included recurrent lymph nodes and recurrent subcutaneous nodules.
Among the 75 cases, 28 cases were excluded including nine patients confirmed as no malignancy after salvage lymphadenectomy; fifteen patients without accurate pathological TNM information; two patients received repeated salvage lymphadenectomy (six times in total). Thus, 47 patients were included in the study at last. All of the patients were restaged according to the 7th edition of AJCC/UICC TNM staging system (5,10).
The institutional review board of Fudan University Shanghai Cancer Center approved the use of the database of esophageal carcinoma for the present study (1602156-1). The informed consent was obtained from each patient at the time of admission.
Follow-up and diagnostic methods for recurrences
After the the initial esophagectomy, patients were usually followed up at our outpatient clinic every 3 months in the first 2 years, and every 6 months in the next 3 years. Oncological investigations such as computed tomography (CT) and ultrasonography (US) were conducted in each follow-up. Upper gastrointestinal endoscopy was arranged annually. For patients with particular symptoms and signs, additional examinations would be conducted to determine whether recurrence existed, including positron emission tomography (PET) and single photon emission computed tomography (SPECT). Fine needle aspiration (FNA) was used for cervical nodes. Usually, surgeons in our center evaluated the operability based on contrast-enhanced CT which provided high spatial resolution.
For the patients included in the survival analyses, a combination of clinical service records, phone calls, and letters was used to determine the status as of April 30th, 2017.
Surgical procedure
The patient was placed in the supine position with his or her head back to exposure cervical and supraclavicular regions. Cervical and cervicothoracic recurrences were dissected as in the procedure of three-field of lymphadenectomy (3FLND) (7). Due to the high rate of involvement (7,11,12), left and right supraclavicular nodes (Group104L and Group104R) and recurrent nerve nodes (Group106recL and Group106recR) were grouped as the four main sites. The other enlarged nodes were regarded the 5th site. Subcutaneous nodules were classified as the 6th site. For patients with suspicious nodes in the unilateral neck, it was depended on the surgeons’ experience and preference to determine whether to perform unilateral or bilateral incisions. Pathologic examination of the specimen was conducted to confirm whether there was malignancy.
Adjuvant treatment after salvage lymphadenectomy
There was no consensus on adjuvant treatment after salvage lymphadenectomy (4,5,13). In our center, the treatment was depended on the surgeon’s judgment and patient’s physical condition and desire. Usually, patients were recommended to receive chemo- or chemoradiotherapy, especially for those with tumor residual.
Statistical analyses
Statistical analyses were performed by Statistical Package for the Social Sciences software (IBM SPSS version 22, Chicago, IL, USA). The survival curve was calculated by the Kaplan-Meier method. The log-rank test was used for univariate analyses and Cox proportional hazards model was used for multivariate analyses. Statistical analysis was considered to be significant when the probability value (P value) was less than 0.05 (P<0.05) . In the present study, the disease-free survival (DFS) was defined as the interval between the date of esophagectomy, or the date of neoadjuvant therapy, and the date of relapse. The post-salvage lymphadenectomy overall survival (PSL-OS) was defined as the interval between the date of the first salvage lymphadenectomy and the date of death or the last follow-up.
Results
Perioperative information of initial esophagectomy
According to the inclusion and exclusion criteria, a total of 47 patients were analyzed in the study. Perioperative information of the initial esophagectomy was shown in Table 1. There were 37 male patients and 10 female patients. The mean age was 56.64±7.84, ranging from 32 to 72. There were eight patients underwent 3FLND. Squamous cell carcinoma was the predominant pathological type, which accounted for 97.8% (46/47). The average number of harvested nodes was 27.72±14.87, with the median number as 24. The average number of positive nodes was 1.87±2.95. There were 24 patients in N0 category. All of the patients had R0 resection. Three patients were at Stage IV due to supraclavicular node involvement while one patient was staged at ypTisN0M0 category. There were 44.7% of patients with lymphatic vessel (LVI) involvement.

Full table
Perioperative information of salvage lymphadenectomy
After initial esophagectomy, patients were followed up as the protocol. Perioperative information of the secondary salvage lymphadenectomy was shown in Table 2. The median DFS was 8 months, ranging from 3 to 65 months. There were 28 patients underwent bilateral cervical incision (namely collar incision) while 19 patients underwent unilateral incision. For the whole 47 patients, the average number of harvested nodes was 13.81±9.82, ranging from 0 to 40; the average number of total positive nodes was 2.77±3.14, ranging from 1 to 14. There were 6 patients had a chief complaint of subcutaneous nodules in the neck: 5 of them received only nodule dissection. Nine (19.1%) patients were considered to have positive margin because of the macroscopic adhesion to trachea, esophagus or other organs and tissues. With regard to the specimen examination, what was notable was that one patient with esophageal squamous cell carcinoma was confirmed adenocarcinoma metastasis to Group104R together with squamous cell carcinoma metastasis to Group106recR. Unfortunately, the patient died in the fourth month after the procedure.

Full table
Left and right supraclavicular nodes (Group104L and Group104R) and recurrent nerve nodes (Group106recL and Group106recR) were grouped as the four main sites. The other enlarged nodes and subcutaneous nodules were named as the 5th site and the 6th site, respectively. The distribution of numbers of positive nodes and the number of positive sites were shown in Table 2. There were 19 patients had only one positive node, and 21 patients had only one positive site. Forty-three patients had lymph nodes recurrences while six patients had subcutaneous nodules recurrences (tissue involvement). Two patients had both recurrent nodes and recurrent subcutaneous nodules. With regard to the other four patients with metastatic subcutaneous nodules, it was remarkable that all the four patients had received 3FLND. With respect to the postoperative events, there was no in-hospital mortality. One patient had postoperative lymphatic fistula. Three patients had postoperative hoarseness.
PSL-OS and prognostic factors
After the salvage lymphadenectomy, the median follow-up was 38 month (95% CI 28.468–47.532). The PSL-OS was shown in Figure 1. The median PSL-OS was 40 months (95% CI 8.850–71.150). The 1-, 2-, 3- and 5-year PSL-OS rate were 87%, 58%, 52% and 41%, respectively.
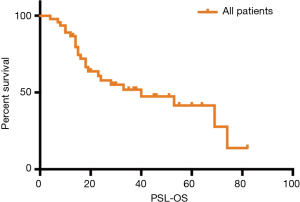
We performed univariate analyses (Log-rank test) and multivariate analyses (Cox proportional hazards model) for PSL-OS. The outcomes were shown in Table 3. Univariate analyses suggested that TNM category (0/I/II vs. III/IV, P=0.000), DFS category (≤8 vs. >8 months, P=0.017) and Number of positive sites (N=1 vs. N>1, P=0.028) were potential prognostic factors. Multivariate analyses revealed that TNM category was the only one independent prognostic factor (P=0.009, HR 3.999, 95% CI: 1.413–11.316). Tumor location was not a prognostic factor (Upper vs. Middle and Lower, P=0.589). Among patients with one positive node (N=1), with at least two positive nodes (N>1) and with tissue involvement, there was no significant survival difference (P=0.557). Patients with initial 3FLND did not show significantly better PSL-OS (P=0.870). The residual status of the salvage lymphadenectomy was not significantly associated with PSL-OS (P=0.065). Patients received post-operative adjuvant therapy (POAT) did not show a significantly better PSL-OS than the counterparts (P=0.056).

Full table
Discussion
Despite the efforts of extended lymphadenectomy, locoregional recurrences are often observed (2,3). A randomized clinical trial reported that after a minimum follow-up of 24 months for surviving patients, mediastinal relapses occurred in 20.5% of patients in the surgery arms; with respect to supraclavicular relapses, the proportion was 4.3% (2). Nakagawa et al. reported that thirty patients (17.5%) developed locoregional recurrences in 174 patients underwent 3FLND for ESCC (3). In their study, locoregional recurrences included recurrences at the site of the primary tumor, at the anastomosis and at the lymph nodes.
The strategy for locoregional recurrences had not been completely established yet (4-6). Usually, patients with locoregional recurrences were thought to have a poor prognosis. Thus, chemo- or chemoradiotherapy was more often applied. According to Nakagawa and his colleagues’ report, among the 10 patients with locoregional recurrences, only one patient received salvage lymphadenectomy for cervical recurrent nodes (3).
The procedure of salvage lymphadenectomy has been performed routinely in our center as the routine application of 3FLND. During the studied period, there were nine patients with false positive nodes. The relatively high rate (9/75, 12.0%) could be partially due to the difficulty of clinical diagnosis as Nakajima and his colleagues’ reported (14). Besides, the 75 patients underwent immediate salvage lymphadenectomy instead of delayed dynamic follow-up. It could also be attributed to the deliberate lymphadenectomy, which worked as a diagnostic method. It was notable that among the 47 patients included in the survival analyses, one patient was confirmed with adenocarcinoma metastasis to Group104R together with squamous cell carcinoma metastasis to Group106recR. The phenomena suggested the necessity of salvage lymphadenectomy for accurate pathology and precise treatment. Analyses on the six patients with metastatic subcutaneous nodules and the eight patients underwent initial 3FLND suggested that there was an overlap of four patients between the groups. For the four patients, the DFS ranged from 7 to 21 months. The PSL-OS ranged from 4 to 64 months. More attention should be paid to the management of cervical incision in case of potential iatrogenic infiltration.
Nakamura et al. reported that the patients received salvage lymphadenectomy had a trend of better survival than those received salvage chemoradiotherapy (13). A previous study from our center suggested that patients underwent salvage lymphadenectomy had a significantly better survival than those underwent salvage chemoradiotherapy, though the P value was marginal (P=0.0467) (9). In our study, for the total 47 patients, the 3-year PSL-OS rate was 52%, which was comparable with the previous study (9). Actually, due to the limited patients’ number, PSL-OS varied a lot in different studies. In 2006, Yano et al. reported 3-year PSL-OS rate as 17.7% after cervical salvage lymphadenectomy (15). In 2008, Nakamura et al. reported that 3-year PSL-OS rate as about 50% for patients with cervical recurrence (13). And they found patients with cervical and mediastinal recurrences seemed to have better PSL-OS. In 2014, Watanabe et al. reported that for patients underwent salvage lymphadenectomy for recurrences after esophagectomy, the 3-year PSL-OS was 75.5% (16). They also found that patients with cervical recurrences had better PSL-OS than those with mediastinal and abdominal recurrences (P=0.0097). Salvage thoracotomy and laparotomy were regarded as much more difficult and invasive due to the adhesion caused by the initial surgery and adjuvant radiotherapy (14).
Univariate and multivariate analyses eventually revealed that only the initial TNM category was the independent prognostic factor for PSL-OS (P=0.000 by log-rank test, P=0.009 by Cox hazards model). According to previous studies, the solitary node recurrence was thought be independently associated with better overall survival (OS) (13,14). However, in our study, statistical analyses revealed that the number of positive nodes (N=1 vs. N>1 vs. tissue involvement, P=0.557) and the number of positive sites (N=1 vs. N>1, P=0.028 by log-rank test, P=0.314 by Cox hazards model) were not independent prognostic factors, neither. The difference deserved deep discussion. Most of the studies included patients with cervical, thoracic and abdominal recurrences. In Yano and his colleagues’ center, only patients with solitary cervical relapse were the candidates for the procedure (15). We supposed the different inclusion criteria could partially contribute to the inconsistence. What’s more, according to previous investigation, lymph node metastasis along the recurrent nerve chain was thought to be an indication for cervical node dissection in thoracic esophageal cancer (17). It meant there was tight connection between the cervical and cervicothoracic regions. Thus, we presumed that there could be no distinct difference between solitary and multiple node recurrences in the limited region. The study was conducted among the patients received similar intervention, thus, we thought it was reasonable to conclude that TNM category was the independent prognostic factor.
With respect to the role of adjuvant therapy after salvage lymphadenectomy, there was no consensus. In Nakajima and his colleagues’ report, patients with R0 resection did not receive adjuvant therapy (14). However, Watanabe et al. hold that patients with recurrences need adjuvant chemotherapy after salvage lymphadenectomy (18). In their opinion, node recurrence was an expression of systematic relapse. The previous study from our center suggested that there was no significant difference between the patients underwent salvage lymphadenectomy with and without adjuvant therapy (P=0.2093) (9). In our study, we found that after salvage lymphadenectomy, patients with adjuvant therapy did not have a survival benefit (P=0.393). The adjuvant therapy included chemo- and chemoradiotherapy. Patients with R1/2 were also included in the analyses. In theory, we presumed that salvage lymphadenectomy followed by adjuvant therapy could be a better approach.
The retrospective has inherent biases. During the studies period, about 4,000 patients received esophagectomy in our center. The study only included patients underwent salvage lymphadenectomy in our center. Thus, there was a selection biases. Besides, in our center, salvage thoracotomy and laparotomy for thoracic and abdominal recurrences were seldom conducted. Thus, the study could not evaluate the role of salvage lymphadenectomy for these patients. What’s more, the study did not compare the efficiency of salvage lymphadenectomy and salvage chemo-/chemoradiotherapy with updated information. It was partially due to the fact of false positive findings. However, the study was aimed to present the outcomes of salvage lymphadenectomy. It would be helpful for further recognition of the procedure.
In conclusion, the study presented outcomes of salvage lymphadenectomy for cervical and cervicothoracic recurrence after esophagectomy. Salvage lymphadenectomy could achieve the precise diagnosis. PSL survival could be considerable, especially for those with early initial tumor stage. Prospective studies are warranted to clarify the value of salvage lymphadenectomy.
Acknowledgements
We gratefully acknowledge the valuable cooperation of Ben Ma (Department of Head and Neck Surgery, Fudan University Shanghai Cancer Center, Shanghai, China) and Xiao Ma (Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of Fudan University Shanghai Cancer Center approved the use of the database of esophageal carcinoma for the present study (1602156-1). The informed consent was obtained from each patient at the time of admission.
References
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]
- Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11. [Crossref] [PubMed]
- D'Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis 2014;6 Suppl 2:S253-64. [PubMed]
- Ajani JA, D’Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Chen Y, Lu Y, Wang Y, et al. Comparison of salvage chemoradiation versus salvage surgery for recurrent esophageal squamous cell carcinoma after definitive radiochemotherapy or radiotherapy alone. Dis Esophagus 2014;27:134-40. [Crossref] [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Li B, Chen H, Xiang J, et al. Pattern of lymphatic spread in thoracic esophageal squamous cell carcinoma: A single-institution experience. J Thorac Cardiovasc Surg 2012;144:778-85; discussion 785-6. [Crossref] [PubMed]
- Ma X, Zhao K, Guo W, et al. Salvage lymphadenectomy versus salvage radiotherapy/chemoradiotherapy for recurrence in cervical lymph node after curative resection of esophageal squamous cell carcinoma. Ann Surg Oncol 2015;22:624-9. [Crossref] [PubMed]
- Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin 2013;23:461-9. [Crossref] [PubMed]
- Japan Esophageal S. Japanese Classification of Esophageal Cancer, 11th edition: part I. Esophagus 2017;14:1-36.
- Mizutani M, Murakami G, Nawata S, et al. Anatomy of right recurrent nerve node: why does early metastasis of esophageal cancer occur in it? Surg Radiol Anat 2006;28:333-8. [Crossref] [PubMed]
- Nakamura T, Ota M, Narumiya K, et al. Multimodal treatment for lymph node recurrence of esophageal carcinoma after curative resection. Ann Surg Oncol 2008;15:2451-7. [Crossref] [PubMed]
- Nakajima M, Domeki Y, Satomura H, et al. Salvage lymphadenectomy for recurrent esophageal cancer after chemoradiotherapy. Int Surg 2014;99:452-7. [Crossref] [PubMed]
- Yano M, Takachi K, Doki Y, et al. Prognosis of patients who develop cervical lymph node recurrence following curative resection for thoracic esophageal cancer. Dis Esophagus 2006;19:73-7. [Crossref] [PubMed]
- Watanabe M, Mine S, Yamada K, et al. Outcomes of lymphadenectomy for lymph node recurrence after esophagectomy or definitive chemoradiotherapy for squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg 2014;62:685-92. [Crossref] [PubMed]
- Shiozaki H, Yano M, Tsujinaka T, et al. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus 2001;14:191-6. [Crossref] [PubMed]
- Watanabe M, Nishida K, Kimura Y, et al. Salvage lymphadenectomy for cervical lymph node recurrence after esophagectomy for squamous cell carcinoma of the thoracic esophagus. Dis Esophagus 2012;25:62-6. [Crossref] [PubMed]


