The long-term outcome of adjuvant hypofractionated radiotherapy and conventional fractionated radiotherapy after breast-conserving surgery for early breast cancer: a prospective analysis of 107 cases
Introduction
For patients with early-stage breast cancer, breast-conserving surgery followed by conventional fractionated radiotherapy of the breast region is a standard therapeutic procedure. However, conventional fractionated radiotherapy is normally delivered for 6–7 weeks, which is not convenient. To address this problem, we cautiously evaluated the use of hypofractionated radiotherapy (HF), which has a shortened course of treatment.
Methods
Study participants
In the present study, 107 female patients, who were all aged over 18 years, underwent breast-conserving surgery. These patients with invasive cancer (tumor staging of pT1-2, pN0-1, and pMx) had a negative surgical margin. The adjuvant systemic therapy included hormonal therapy, targeted therapy, and systemic chemotherapy. The presence of signs and symptoms that are associated with chemotherapy indicated that the participant previously underwent systemic chemotherapy. If the breast cancer is HER2 (+), the part of participant should receive Herceptin treatment. The exclusion criteria were as follows: (I) previous diagnosis of malignant cancer, including breast cancer (n=2); (II) bilateral breast cancer (n=2); (III) breast cancer during pregnancy and lactation (n=0); (IV) cancer with co-occurrence of cardiovascular and benign pulmonary diseases that affect normal radiotherapy (n=2); (V) previous history of mental illness (n=1); (VI) recent chemotherapy making the administration of radiotherapy impossible after less than 3 weeks from the last chemotherapy treatment (n=1); and (VII) previous adjuvant chemotherapy before breast-conserving surgery (n=1). The institutional ethical committee approved the present study, and all patients provided their informed consent forms (ICFs).
Breast-conserving surgery
Breast-conserving surgery involved the local resection of breast tumors and management of regional lymph nodes. The local resection of breast tumors refers to the complete excision of visible tumors and the surrounding 1 cm margin of normal breast tissue. During the operation, 4–6 radiopaque titanium clips for part patients were placed to mark the surgical edge, thereby providing reference points for positioning patients during radiotherapy in the future. The management of regional lymph nodes included the guided biopsy of sentinel lymph nodes or dissection of lymph nodes in the axilla (levels I and II).
CT-simulated positioning
Each patient was asked to lie in a supine position for examination using a wing-board simulation localization system (Brilliance CT Big Bore, Philips Medical Systems, USA), during which a mammary bracket was utilized. The ipsilateral arm was raised above the head. Each participant underwent free-breathing scanning, and the following settings were used: slice thickness of 5 mm, interslice gap of 5 mm, and a scanning range from the middle of the inferior maxillary bone to 5 cm below the lower breast edge.
Delineation of the target
A 3D treatment system (Pinnacle3 8.0 version, Philips Medical Systems, USA) was used for all patients who underwent radiotherapy treatment, which was used to delineate the target and design of the radiotherapy plan. (I) Whole breast: the clinical target volume (CTV) within the ipsilateral breast volume, excluding the chest wall and 0.5 cm from the skin. The planning target volume (PTV) expanded the CTV with a 5-mm margin (10 mm in a cephalocaudal trend, not in the direction of the skin); (II) tumor bed: the surgical cavity was delineated using the intraoperatively introduced titanium clips. If the clips were not utilized, the tissue defect from the surgery and its scar and reference preoperative imaging with an adequate margin were used to delineate the field (1-3). The CTV expanded the surgical cavity with a 10-mm margin. The PTV expanded the CTV with a 5-mm margin, and it was adjusted according to anatomical position; (III) infra-supraclavicular region: the CTV covered the ipsilateral infra-supraclavicular region. The PTV expanded the CTV with a 5-mm margin. The organs that were at risk included the heart and lungs. The spinal cord, contralateral breast, trachea, brachial plexus, and humeral head may also be affected.
Irradiation mode
Whole-breast and infra-supraclavicular region irradiation (6 MV X-ray) involved irradiation with intensity-modulated radiation therapy, during which lung exposure was minimized. The tumor bed was treated with 6-MV X-ray irradiation with intensity-modulated radiation therapy or 6–15-MeV electron beam irradiation at an appropriate energy level (the N1 patient received irradiation of the infra-supraclavicular region). The radiotherapy device was a linear accelerator (Clinac 21EX, Varian Medical Systems, USA).
Plan assessment
Treatment was performed with a dose range of PTV with 95–105% of the prescribed dose, For the organs at risk and their dose limits see Table 1.
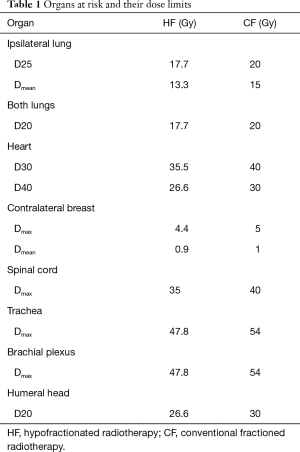
Full table
Radiation dose and fractionation mode
For the HF group, whole-breast irradiation (± infra-supraclavicular region) required a dose of 42.56 Gy/16 fractions, followed by a tumor bed boost of 7.98 Gy/3 fractions. A total of 19 irradiation fractions were delivered (2.66 Gy/fraction), which were performed daily from Monday to Friday every week and accomplished within 25–27 days/4 weeks.
For the conventional fractionated (CF) group, whole-breast irradiation (± infra-supraclavicular region) required a dose of 50 Gy/25 fractions, followed by a tumor bed boost of 10 Gy/5 fractions. A total of 30 irradiation fractions (2.0 Gy/fraction) were delivered, which were performed daily from Monday to Friday every week and accomplished within 40–42 days/6 weeks.
Follow-up and cosmetic assessment
After radiotherapy, patients were monitored once every 3 months for 2 years and then once every 6 months for 3 years, after which a follow-up visit was conducted once every year. The examination items included routine disease history collection and physical examination, bilateral breast X-ray or MRI, chest CT scanning, routine blood examination, liver and kidney function tests, and abdominal B ultrasound or CT scan. If the participant presented with any discomfort, the schedule of the follow-up visit was adjusted accordingly. Only one patient was not followed-up (109 months), according to the truncated value (end) follow-up processing. The follow-up rate was 99.1%. The primary outcome was local recurrence (LR, i.e., local tumor occurrence + regional occurrence). Local tumor recurrence referred to ipsilateral local relapse within the irradiated breast region, whereas regional relapse referred to ipsilateral regional relapse in the axilla or supraclavicular fossa or internal mammary lymph if it had been within an irradiated target volume. Any ipsilateral regional relapse outside the radiotherapy target volume was excluded from the analysis of local regional relapse (4). The secondary outcomes were distant metastasis and death, which were based on the tumor-specific survival rate, disease-free survival rate, and overall survival rate. To calculate the tumor-specific survival rate, all data related to breast cancer (e.g., LR, distant metastases, development of contralateral breast cancer, or death resulting from breast cancer) were compiled. To calculate the disease-free survival rate, all data about the disease were compiled. To calculate the overall survival rate, all data on patient death were compiled.
The third outcome was the breast cosmetic result (before radiotherapy and 5 and 10 years after radiotherapy) and delayed radioactive toxic reactions (5 and 10 years after radiotherapy). The assessment was conducted by two highly trained senior nurses (the nurse is trained once a year), and if the two assessments had different results, a reassessment was performed. In addition, during the evaluation of the clinical trial results, the nurses did not know the treatment assignment to reduce assessment bias. The assessment of the cosmetic outcome was performed according to the criteria proposed by Harris et al. (5), in which the appearance of the breast was graded according to 4 levels: excellent, good, fair, and poor. The 4 levels are defined as follows: excellent, the treated breast showed little changes or no change; good, the treated breast showed no significant changes; fair, the treated breast showed significant changes; and poor, the treated breast showed serious changes. Toxic effects were evaluated based on the criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) (6,7). The cosmetic outcomes and toxic effects were not evaluated in patients who experienced LR.
Statistical methods
In the current study, 107 patients were recruited in 2 years. We did make an estimation of sample size according to the published similar studies (8,9). The χ2 test or Fisher’s exact test was performed to compare the clinically categorized data, breast cosmetic results, and delayed toxic effects observed in the two groups, and the t-test was carried out to analyze the age groups. A propensity score matched analysis (PSM) is conducted for increasing confidence in study conclusion using STATA. The Kaplan-Meier method was used to calculate the LR rate, tumor-specific survival rate, disease-free survival rate, and overall survival rate, and the log-rank test was used to examine inter-group differences. The hazard ratio (HR) with its corresponding 95% confidence interval (CI) was obtained based on the Cox proportional hazard model, with adjustment for potential confounders, such as tumor-specific survival rate. In addition, the uses of score test to the proportional hazards assumption, P value less than 0.05. SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Population characteristics
Between January 2006 and December 2007, 107 patients treated at Shaanxi Provincial People’s Hospital met our recruitment criteria. These patients were randomly divided into the HF group (53 participants) and the conventional fractioned radiotherapy (CF) group (54 participants). Follow-up visits were conducted until December 2016, with a median follow-up time of 122 months (108–132 months). The two groups had similarities in terms of various clinical parameters, including age, tumor staging, lymph node staging, pathological type, hormone status, Her2 expression, and side of the tumor (Table 2). In addition, we have tried to conduct a PSM analysis, and the results did not change. In the analysis, there were five cases in CF group that failed to match to the appropriate control, the matching rate was 95.33%.
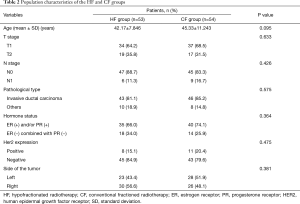
Full table
LR
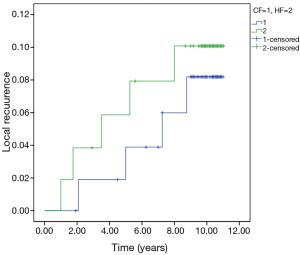
Eight cases of local tumor recurrence and 1 case of regional recurrence (4 cases of local tumor recurrence and 1 case of recurrence in the supraclavicular lymph nodes in the HF group and 4 cases of local tumor recurrence in the CF group) were observed. The 5- and 10-year cumulative LR rates of the HF and CF groups were 5.7% vs. 3.9% and 9.6% vs. 7.9%, respectively (P=0.712) (Figure 1).
Cases of distant metastasis of tumors and death
In the present study, a total of 13 distant metastatic cases were observed, of which 7 occurred in the HF group (including 1 case of co-occurrence with LR) and 6 occurred in the CF group (including 1 case of co-occurrence with LR).
Thirteen death cases were observed (7 and 6 occurred in the HF group and CF group, respectively). The 7 death cases in the HF group comprised 6 cases of death due to the tumor and 1 death due to other diseases. The 6 death cases in the CF group comprised 5 cases of death due to the tumor and 1 death due to cardiovascular diseases. No cases of contralateral breast cancer were observed during this study.
Tumor-specific survival rate, disease-free survival rate, and total survival rate
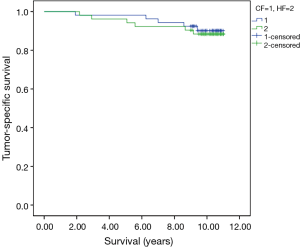
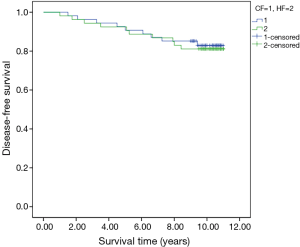
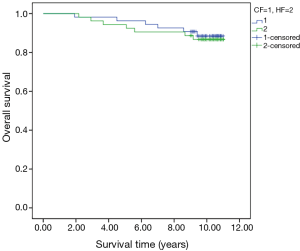
The 5- and 10-year tumor-specific survival rates of patients in the HF and CF groups were 96.2% vs. 98.1% and 88.1% vs. 90.1%, respectively (P=0.738) (Figure 2). The 5- and 10-year disease-free survival rates of patients in the HF and CF groups were 92.5% vs. 90.7% and 81.1% vs. 82.9%, respectively (P=0.792) (Figure 3). The 5- and 10-year overall survival rates of patients in the HF and CF groups were 94.3% vs. 96.3% and 86.5% vs. 88.5%, respectively (P=0.748) (Figure 4).
Assessment of cosmetic and delayed toxic effects
The two groups were classified into “excellent + good” group and “fair + poor” group based on cosmetic effects using the bisection method. Delayed radioactive toxic effects were divided into “Grade 0” group and “Grade 1, 2 and 3” groups.
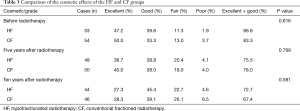
Before radiotherapy, the rates of the patients with excellent and good cosmetic results in the HF and CF groups were 86.8% vs. 83.3%, respectively (P=0.616). The corresponding 5- and 10-year rates of the patients in the two groups after radiotherapy were 75.5% vs. 78.0% (P=0.769) and 72.7% vs. 67.4% (P=0.581) (Table 3).
Full table
The 5- and 10-year rates of the patients with toxicity-free skin (Grade 0) in the HF and CF groups after radiotherapy were 77.6% vs. 80.0% (P=0.766) and 70.5% vs. 65.2% (P=0.595). No cases of stage IV skin ulcers were observed during this study (Table 4).

Full table
The 5- and 10-year rates of the patients with toxicity-free subcutaneous tissues (Grade 0) in the HF and CF groups were 67.3% vs. 64.0% (P=0.726) and 52.3% vs. 47.8% (P=0.673). No cases of stage IV subcutaneous tissue necrosis were observed during this study (Table 5).

Full table
Analysis of the 10-year tumor-specific survival rate using the Cox risk regression model
Before the analysis using the Cox risk regression model, any potential confounders were analyzed and excluded as inclusion and exclusion criteria. A total of 9 patients were excluded. The breast cancer specialist was responsible for guiding surgery and controlling the quality during radiotherapy. A qualified oncologist was responsible for making chemotherapy and other systematic treatment.
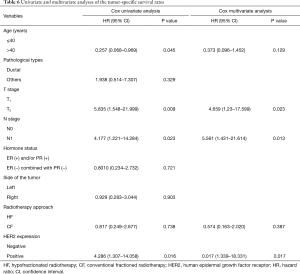
Multiple clinical parameters and the radiotherapy approach (for the HF and CF group), which may affect the tumor-specific survival rate, were analyzed using the Cox univariate regression model. Results showed that age, T stage, N stage, and HER2 expression were the prognostic factors that affected the 10-year tumor-specific survival rate of the patients, all of which were statistically significant (P<0.05). The above-mentioned parameters were statistically significant, and the radiotherapy approach (for the HF and CF group) was further evaluated via a multivariate analysis using the Cox regression model. Results showed that T stage (P=0.023), N stage (P=0.013), and HER2 expression (P=0.017) were the independent prognostic factors that affected the 10-year tumor-specific survival rate of patients (Table 6).
Full table
Discussion
The goal of adjuvant radiotherapy is to reduce the LR rates, minimize irradiation-associated toxic effects, and obtain an accepted cosmetic outcome after breast-conserving surgery (10). The standard regimen of the conventional fractionated radiotherapy is as follows: 1 fraction/day, 1.8–2.0 Gy/fraction for 5 days/1 week. Based on the secondary linear mathematical model of radiobiology, the biological effects of radiation equivalents are directly dependent on the total radiation dose and single dose. Because breast cancer tissues have a comparable a/ß ratio with normal tissues, treatments with a large single dose and reduced total dose may theoretically have acceptable effects compared with conventional fractionated treatment (11,12). In previous studies about HF, different plans for hypofractionation have been undertaken. In addition, studies have been performed to address whether tumor bed boost should be used after whole-breast irradiation.
Based on long-term staging analyses of (4,13,14) experiments in the UK in which three regimens were tested (3.0 Gy/fraction and 3.2 Gy/fraction in trial A and 2.67 Gy/fraction in trial B), the three HF regimens generated comparable results compared to conventional fractionated treatment. Likewise, Owen et al. (15) reported that HF regimens of 3.0 Gy/fraction and 3.3 Gy/fraction showed similar long-term outcomes to conventional fractionated treatment. In China, studies on the long-term outcome of HF of the whole breast after breast-conserving surgery are not available.
In the present study, a total of 107 participants (in China) were recruited and randomly classified into two groups, which have comparable clinical data (Table 2). Our results revealed that the HF group (2.66 Gy/fraction) and the CF group (2.0 Gy/fraction) did not show significant differences in terms of the 5- or 10-year LR rates, tumor-specific survival rates, disease-free survival rates, and overall survival rates. In addition, the two groups had similar results with respect to delayed toxic effects, with no significant differences in the delayed toxic effects in the skin or subcutaneous tissues. In the present study, no cases of delayed toxic effects in the lungs were identified. This result is probably due to the small number of N1 patients (6 cases in the HF group and 9 cases in the CF group), thus only few participants received infra-supraclavicular region treatment that resulted in the irradiation of the pulmonary apex. In addition, no obvious fibrosis was observed in the infra-supraclavicular region and brachial plexus, and trachea-related injury was not noted as well. However, only 15 patients underwent irradiation of the infra-supraclavicular region (6 women in the HF group and 9 women in the CF group), and fewer cases were observed, thus further large-scale studies might be performed. Overall, our results revealed that HF showed a comparable long-term efficacy and similar delayed toxic effects to conventional fractionated treatment. Radiotherapy can cause telangiectasia and thickening of the subcutaneous tissues, which cause poor breast cosmetic effects (16). Hence, in addition to the examination of its clinical efficacy, we analyzed the breast cosmetic effects in the two groups. Results showed that the 5- and 10-year rates of the patients with excellent and good cosmetic effects in the two groups were comparable. Moreover, since the follow-up duration was extended, the rates of the patients with excellent and good cosmetic effects in both groups showed a slight reduction but remained acceptable.
Results of the subsequent univariate and multivariate regression analyses indicated that the 10-year tumor-specific survival rate was correlated with age and tumor status in participants aged ≤40 years and relatively >40 years who have a relatively poor prognosis (T2 stage, relative to T1 stage; N1 stage, relative to N0 stage; and HER2 positive, relative to HER2 negative), and the later three factors are independent prognostic factors (Table 6). The multivariate regression analyses indicated no statistically significant difference based on age stratification (P>0.05), which is, younger patients chose breast conservation more than older patients to satisfy their cosmetic and mental needs. Older patients prefer total mastectomy. About 81.3% of patients in this group were ≤50 years old. The proportion of older patients was extremely small, and the age difference was also significantly small. No statistical difference was observed in age stratification. In addition, HER2 expression is also one of the factors that affects prognosis, which can be improved with the use of the molecular-targeted drug Herceptin. In this study, 8 HER2 (+) cases were observed in the HF group, of which 2 received Herceptin treatment. Moreover, 11 HER2 (+) cases were observed in the CF group, of which 3 patients received Herceptin treatment. In total, only 5 HER2 (+) patients received Herceptin treatment, which might account for the fact that HER2 expression did affect the prognosis based on the risk factor analysis.
Several issues regarding the long-term cardiovascular effects and suitability of HF for G3 tumors were discussed. A previous study on HF (40–44 Gy/16 fractions) and conventional fractionated radiotherapy (45–50 Gy/25 fractions) revealed that no significant differences were observed in the two groups in terms of cardiovascular diseases after 15 years of long-term follow-up visits (17,18). During the follow-up examination in our study period, few cases of patients with adverse cardiovascular reactions were observed, of which one patient died in the CF group. In future follow-up visits, adverse cardiovascular reactions should be the main focus for 15 years. Tumor pathological staging was not considered when designing the recruitment criteria. The suitability of HF for G3 tumors is the primary issue. A previous retrospective study showed that 10-year LR rates of 6.9% and 6.2% (P=0.99) was observed in patients with G3 early-stage breast cancer who were treated with HF (42.5 Gy/16 fractions/22 days) and conventional fractionated radiotherapy (50 Gy/25 fractions/35 days), respectively (P=0.99) (19). Therefore, HF is suitable for G3 patients. Although no tumor staging was performed in our study, the results were not affected.
Whether additional irradiation from the tumor bed boost has an effect on breast cosmetic effect was evaluated. In Canada, a study showed that whole-breast HF (42.5 Gy/16 fractions/22 days) without tumor bed boost had a similar 10-year efficacy to conventional fractionated treatment (50 Gy/25 fractions/35 days) in treating early-stage breast cancer that was margin negative and lymph node negative after breast-conserving surgery. For example, the 10-year LR rates of the HF and CF groups were 6.2% and 6.7%, respectively, and the 10-year rates of patients with excellent and good cosmetic results in the two groups were 69.8% and 71.3%, respectively (20,21). In the previous study, due to the concern that tumor bed boost might compromise the aesthetic outcome in the breast, this step was not performed. However, a randomized trial in Australia revealed that the application of either a regimen of the whole-breast irradiation at 45–50 Gy followed by a tumor bed boost of 16 Gy/8 fractions or a regimen of the whole-breast irradiation at 45–50 Gy without a tumor bed boost showed no significant difference in the 5-year rates of patients with excellent and good cosmetic results (22). In Japan, clinical studies on boost irradiation of patients with positive margins obtained favorable results without severe toxicity (23), and multiple studies on (24-26) HF followed by a tumor bed boost also had favorable cosmetic effects. In our study that used an additional irradiation from a tumor bed boost also obtained favorable cosmetic outcomes.
In addition, Doré et al. (27) and Cante et al. (25) revealed that whole-breast HF after breast-conserving surgery is a safe and efficient approach for elderly patients.
In summary, HF had a comparable efficacy to conventional fractional radiotherapy in treating patients with early-stage breast cancer. However, HF is advantageous because it has shorter treatment time and fewer radiotherapy fractions, thereby reducing patient expenditures (28) and conserving medical resources. Thus, HF is more convenient for patients who want to seek for medical attention and improve their radiotherapy compliance. Furthermore, HF can be an alternative for conventional fractionated radiotherapy.
Acknowledgements
Funding: The project was supported by the Programs for Scientific and Technological Development of Shaanxi Province, China {Project No. 2007k09-06 [2]}.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: The study was approved by the Ethics Committees of Shaanxi Provincial People’s Hospital (No. 2007002), and a written informed consent was obtained from all patients.
References
- Kim JH, Choi DH, Park W, et al. Influence of boost radiotherapy in patients with ductal carcinoma in situ breast cancer: a multicenter, retrospective study in Korea (KROG 11-04). Breast Cancer Res Treat 2014;146:341-5. [Crossref] [PubMed]
- Meattini I, Livi L, Franceschini D, et al. Role of radiotherapy boost in women with ductal carcinoma in situ: a single-center experience in a series of 389 patients. Eur J Surg Oncol 2013;39:613-8. [Crossref] [PubMed]
- Kyrgias G, Zygogianni A, Theodorou K, et al. Accelerated hypofractionated whole-breast irradiation with concomitant daily boost in early breast cancer. Am J Clin Oncol 2015;38:358-63. [Crossref] [PubMed]
- START Trialists’ Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371:1098-107. [Crossref] [PubMed]
- Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257-61. [Crossref] [PubMed]
- Rubin P, Constine LS, Fajardo LF, et al. RTOG Late Effects Working Group. Overview. Late Effects of Normal Tissues (LENT) scoring system. Int J Radiat Oncol Biol Phys 1995;31:1041-2. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Amouzegar Hashemi F, Barzegartahamtan M, Mohammadpour RA, et al. Comparison of Conventional and Hypofractionated Radiotherapy in Breast Cancer Patients in Terms of 5-Year Survival, Locoregional Recurrence, Late Skin Complications and Cosmetic Results. Asian Pac J Cancer Prev 2016;17:4819-23. [PubMed]
- Hou HL, Song YC, Li RY, et al. Similar Outcomes of Standard Radiotherapy and Hypofractionated Radiotherapy Following Breast-Conserving Surgery. Med Sci Monit 2015;21:2251-6. [Crossref] [PubMed]
- Luini A, Gatti G, Galimberti V, et al. Conservative treatment of breast cancer: its evolution. Breast Cancer Res Treat 2005;94:195-8. [Crossref] [PubMed]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679-94. [Crossref] [PubMed]
- Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol 2005;44:265-76. [Crossref] [PubMed]
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086-94. [Crossref] [PubMed]
- START Trialists’ Group, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 2008;9:331-41. [Crossref] [PubMed]
- Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol 2006;7:467-71. [Crossref] [PubMed]
- Hamilton CS, Nield JM, Adler GF, et al. Breast appearance and function after breast conserving surgery and radiotherapy. Acta Oncol 1990;29:291-5. [Crossref] [PubMed]
- Chan EK, Woods R, McBride ML, et al. Adjuvant hypofractionated versus conventional whole breast radiation therapy for early-stage breast cancer: long-term hospital-related morbidity from cardiac causes. Int J Radiat Oncol Biol Phys 2014;88:786-92. [Crossref] [PubMed]
- Chan EK, Woods R, Virani S, et al. Long-term mortality from cardiac causes after adjuvant hypofractionated vs. conventional radiotherapy for localized left-sided breast cancer. Radiother Oncol 2015;114:73-8. [Crossref] [PubMed]
- Herbert C, Nichol A, Olivotto I, et al. The impact of hypofractionated whole breast radiotherapy on local relapse in patients with Grade 3 early breast cancer: a population-based cohort study. Int J Radiat Oncol Biol Phys 2012;82:2086-92. [Crossref] [PubMed]
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst 2002;94:1143-50. [Crossref] [PubMed]
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513-20. [Crossref] [PubMed]
- Hau E, Browne LH, Khanna S, et al. Radiotherapy breast boost with reduced whole-breast dose is associated with improved cosmesis: the results of a comprehensive assessment from the St. George and Wollongong randomized breast boost trial. Int J Radiat Oncol Biol Phys 2012;82:682-9. [Crossref] [PubMed]
- Ishihara T, Yoden E, Konishi K, et al. Long-term outcome of hypofractionated radiotherapy to the whole breast of Japanese women after breast-conserving surgery. Breast Cancer 2014;21:40-6. [Crossref] [PubMed]
- Ghannam AA, Khedr RA. An accelerated hypofractionated schedule with a daily concomitant boost after breast conservation surgery: the feasibility and toxicity. J Egypt Natl Canc Inst 2016;28:39-44. [Crossref] [PubMed]
- Cante D, Franco P, Sciacero P, et al. Hypofractionated whole-breast radiotherapy and concomitant boost after breast conservation in elderly patients. Tumori 2016;102:196-202. [Crossref] [PubMed]
- Ciammella P, Podgornii A, Galeandro M, et al. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: predictive clinical and dosimetric factors. Radiat Oncol 2014;9:97. [Crossref] [PubMed]
- Doré M, Cutuli B, Cellier P, et al. Hypofractionated irradiation in elderly patients with breast cancer after breast conserving surgery and mastectomy: Analysis of 205 cases. Radiat Oncol 2015;10:161. [Crossref] [PubMed]
- Dwyer P, Hickey B, Burmeister E, et al. Hypofractionated whole-breast radiotherapy: impact on departmental waiting times and cost. J Med Imaging Radiat Oncol 2010;54:229-34. [Crossref] [PubMed]


