Initial experience of medical pleuroscopy via the peel-away introducer of the indwelling pleural catheter using a thin bronchoscope
Introduction
The diagnosis of a cause of pleural effusion is commonly made on clinical grounds in conjunction with findings of thoracentesis. Medical thoracoscopy or medical pleuroscopy (MP) is usually indicated for suspected malignant etiology or exudative pleural effusions which remain undiagnosed in spite of multiple thoracenteses (1). This is particularly important for patients with cancer where accurate determination of pleural metastasis can have a significant impact on staging and treatment plan. In the era of molecular testing, pleural biopsies may be needed for additional tissue even if a diagnosis has been established with pleural fluid cytology (2). Chemical pleurodesis and the placement of indwelling pleural catheters (IPC) are the common therapeutic options for symptomatic pleural effusions (3). For patients who elect outpatient management, pleuroscopy along with IPC placement can be performed in one setting. In this report, we present a case series of nine patients where a new technique combining MP using a thin bronchoscope via the peel-away sheath of IPC followed by IPC placement were successfully performed.
Methods and materials
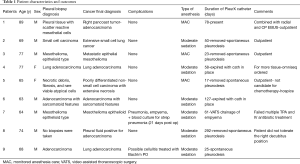
In this case-series, we describe nine patients who presented with recurrent pleural effusions. The patients’ characteristics are presented in Table 1. All nine patients were scheduled to undergo IPC placement and diagnostic pleuroscopy in an outpatient setting. All the procedures were performed after informed consent in the endoscopy suite in strict sterile conditions. Prophylactic antibiotic was given in the preoperative period.
Full table
Procedure
Step one: the IPC placement
The procedure was performed with patients in decubitus position and the ultrasound was used to identify the pleural fluid and mark the chosen area of IPC (PleurXTM, Carefusion, McGaw Park, Illinois, USA) insertion at the posterior, middle or anterior axillary line. Moderate sedation or monitored anesthesia care (MAC) was used in all patients. The marked area was then sterilized using chlorhexidine preparation. About 20 milliliters of lidocaine 1% was used for local anesthesia. The 18 Gauge needle introducer catheter was then used to access the pleural fluid and a flexible guidewire was passed through it and into the pleural space. The introducer catheter was then removed and a 1 cm incision was made at the site of guidewire insertion. Another 1 cm incision was made about two inches inferomedially from the first incision. The metallic tunneler was used to tunnel the fenestrated end of the catheter. The indwelling catheter peel-away introducer sheath and dilator assembly was then inserted over the wire into the pleural space. The guidewire and dilator were removed and the peel-away introducer sheath was left in place.
Step two: medical pleuroscopy
For this step of the procedure, the hybrid bronchoscope (BF-MP160F, Olympus, Japan) was sterilized using the STERRAD 36 NX Sterilization System, a low-temperature hydrogen peroxide gas plasma sterilizer, as per the manufacturer’s recommended protocol.
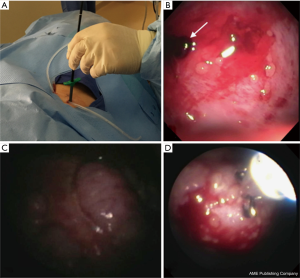
The hybrid bronchoscope with an outer diameter (OD) of 4.0 mm and an inner working channel of 2.0 mm was introduced through the peel away introducer into the pleural place (Figure 1A). The pleural fluid was removed using the bronchoscope suction or a separate suction catheter was introduced though the peel away introducer for faster pleural fluid aspiration. The peel away introducer was gently manipulated to guide the tip of the scope to facilitate the inspection of the pleural cavity. After identifying an accessible target lesion on the parietal pleura, a regular flexible bronchoscopy forceps (1.8 mm diameter, Radial Jaw, Boston Scientific, Boston, MA, USA) was used to obtain pleural biopsies (Figure 1B-D). The bronchoscope was then withdrawn and the pleuroscopy step of the procedure concluded.
Step three: IPC insertion
The fenestrated end of the catheter was then inserted into the pleural space through the peel away introducer. The catheter was then connected to suction to drain the pleural air and remnant fluid. The incision sites were sutured and sterilely dressed. Postoperative chest portable plain film was used to verify the catheter in place and the drainage of most pleural air.
Results
Nine patients were included in this case-series. Seven were male and two were females with age range between 63 and 89 years. Pleural biopsies were performed in eight patients. In one patient (patient 1), there was only minimal inflammation (erythema) of the parietal pleura surrounding the apical Pancoast tumor area as it invaded the chest wall. Pleural biopsies showed scattered reactive mesothelial cells. In another patient (patient 5), large pleural friable tumors were identified and biopsies were easily taken but pathologic examination showed necrotic debris, and rare viable atypical cells. Ultrasound-guided percutaneous lung mass biopsies subsequently showed extensive necrosis and poorly differentiated carcinoma. In the other six patients, the pleural biopsies yielded a diagnosis of cancer. Two of these patients had epithelial mesothelioma and four were lung cancers (Table 1).
In one patient (patient 8), pleural biopsies were not possible, as the patient did not tolerate the right decubitus position. He developed severe dyspnea and desaturation soon after being placed in the right decubitus position. This patient had a large right paratracheal mass with tracheal deviation and compression that was likely worsened by this position. Previously, this patient had thoracentesis showing less than 1% adenocarcinoma cells and the pleural biopsy was requested for more tissue for molecular analysis. The patient was repositioned in the semi-sitting position and the procedure was performed. When the hybrid scope was inserted into the peel-away introducer, the view of the pleural space was limited because of lung expansion due to the sitting position and the patient’s tachypnea and coughing. Despite identifying multiple pleural lesions, the manipulation of the bronchoscope was very challenging and biopsies couldn’t be obtained.
Discussion
In this report, we describe the successful use of an innovative technique that allows the proceduralist to perform pleuroscopy and pleural biopsy using the peel away introducer that comes with the IPC placement kit. The peel away introducer functioned as a trocar to introduce the hybrid bronchoscope thus allowing it to be utilized as a pleuroscope to evaluate the pleural cavity and to obtain pleural biopsies. The above described procedure was successfully performed on eight patients. In one patient, the decubital positioning was not tolerated because of concomitant central airway compression by tumor and therefore, any pleuroscopy technique would not have been possible.
MP has been routinely performed using the flex-rigid or the rigid scope. In both cases, metallic or plastic trocars of different sizes are used to gain access to the pleural cavity. Both pleuroscopy methods have similar safety profile and diagnostic yield (1). Some suggested lower complication rate using the flex-rigid compared to rigid MP (4). Major complications of MP are rare (1.8%) and include empyema, hemorrhage, post site metastasis, broncho-pleural fistula, pneumothorax and pneumonia. Minor complications such subcutaneous emphysema and site infection are more common (7.3%). Overall, the reported mortality rate from MP is very low (0–0.34%) (1,5,6). The available semi-rigid pleuroscope has an OD of 7.0 mm and requires a trocar of at least 8 mm in diameter.
The peel-away introducer of IPC kit is about 15.5 French in diameter, which corresponds to a diameter of 5.17 mm. The hybrid bronchoscope with an OD of 4 mm and a working channel of 2 mm can be easily introduced through the peel away introducer without any resistance without any need for lubrication.
Our technique has multiple advantages and limitations. The most important advantage is the ability to simplify and combine IPC placement and pleuroscopy and pleural biopsy using one technique. This can potentially limit the procedure time and minimize the medical equipment for such combined procedure. Given that IPC placement is been widely performed by general pulmonologists, our technique offers a potentially easier method to combine IPC with MP for pleural biopsy without the need for significant training. Although pain control postoperatively was not evaluated in our case series, none of the patients required hospitalization or significant pain control postoperatively. The incision for inserting the peel-away introducer is smaller than what is required for traditional MP as larger trocars are needed. The use of trocars had been shown to induce pain due to rib retraction and intercostal muscle damage. Placing a larger trocar may lead to more ribs spreading, which may affect postoperative degree of pain (7).
This case series has some limitations. It has a small sample size prospective larger studies are warranted to examine the diagnostic yield, complications, and outcomes of this technique and to compare it to conventional MP modalities.
The other limitation could be the sterilization process of the bronchoscope. The flexible bronchoscopes may be sterilized using different methods such as ethylene oxide gas or hydrogen peroxide gas plasma. MP has been previously described using flexible bronchoscopes with no reported infectious complications (8-10). However, the use of approved sterilization techniques is of crucial importance. To prevent scope damages and to achieve optimal sterilization, the factory manual should always be used for guidance since sterilization methods differ between bronchoscopes and manufacturing companies (11). This could be challenging as some institutions may not possess the appropriate sterilization equipment for the intended bronchoscope. The smaller 2 mm working channel of the used bronchoscope could also pose some limitations as the larger flexible forceps can’t be utilized and it also limits the possibility of any therapeutic interventions.
The absence of a rigid component of the bronchoscope can limit scope manipulation and make pleural biopsies challenging. The light delivered by the hybrid bronchoscope is significantly lower compared to the conventional MP techniques and the images are not as clear as the traditional MP. Some technical limitations can be solved by manufacturing a smaller semi-rigid pleuroscope that had smaller OD and larger inner diameter and brighter light.
Conclusions
This case series describes the feasibility and safety of a modified MP technique in a small group of patients and provides the rationale of performing a larger study to assess this technique further.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alraiyes AH, Dhillon SS, Harris K, et al. Medical Thoracoscopy: Technique and Application. PLEURA 2016;3:2373997516632752. [Crossref]
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010;138:1242-6. [Crossref] [PubMed]
- Porcel JM, Lui MM, Lerner AD, et al. Comparing approaches to the management of malignant pleural effusions. Expert Rev Respir Med 2017;11:273-84. [Crossref] [PubMed]
- Yap KH, Phillips MJ, Lee YG. Medical thoracoscopy: rigid thoracoscopy or flexi-rigid pleuroscopy? Current opinion in pulmonary medicine 2014;20:358-65. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii54-ii60. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D. Diagnostic accuracy and safety of semirigid thoracoscopy in exudative pleural effusions: a meta-analysis. Chest 2013;144:1857-67. [Crossref] [PubMed]
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Yokoyama T, Toda R, Tomioka R, et al. Medical Thoracoscopy Performed Using a Flexible Bronchoscope Inserted through a Chest Tube under Local Anesthesia. Diagn Ther Endosc 2009;2009:394817. [PubMed]
- Williams T, Thomas P. The diagnosis of pleural effusions by fiberoptic bronchoscopy and pleuroscopy. Chest 1981;80:566-9. [Crossref] [PubMed]
- Sarkar SK, Purohit SD, Sharma TN, et al. Pleuroscopy in the diagnosis of pleural effusion using a fiberoptic bronchoscope. Tubercle 1985;66:141-4. [Crossref] [PubMed]
- Harris K, Singh Dhillon S, Alraiyes AH. Medical Pleuroscopy Using a Peel-Away Introducer Sheath and a Hybrid Bronchovideoscope. Ann Am Thorac Soc 2016;13:976-8. [Crossref] [PubMed]


