Not like breast cancer, but like breast cancer: micrometastasis and micropapillary structure in lung cancer
Micropapillary growth pattern is characterized, as morphologic features of small tight clusters of tumor cells floating in clear spaces. Initially, aggressive nature of this pattern has been reported in bladder, prostate and breast cancer (1), and included in lung cancer classification subsequently. The growth pattern is commonly admixed with other growth patterns, and micropapillary pattern is seen only a part of the tumor.
Dai et al. recently evaluated the relationship between lymph node (LN) micrometastasis and histologic patterns of adenocarcinoma (2). LN micrometastasis, defined as isolated tumor cells or cellar clusters <0.2 mm in greatest dimension, which are equivalent to that of breast cancer (2). In this study, LN micrometastasis had a negative influence on recurrence and survival among patients with stage I adenocarcinoma. Furthermore, micrometastasis was more frequently observed in adenocarcinoma with micropapillary component, which is an independent predictor for increased frequency of LN micrometastasis. Therefore, the authors concluded that positive findings of LN micrometastasis in patients with stage I lung adenocarcinoma with a micropapillary component require a sufficient and systematic LN dissection during operation.
According to the 8th TNM staging system, LN metastases are divided into three groups; isolated tumor cell clusters (ITCs), micrometastasis and macrometastases. ITCs are defined as single cells or cell clusters, either measuring <0.2 mm in size or amounting to <200 cancer cells in one LN section, while micrometastasis range in size from 0.2 to 2 mm. Micrometastasis are tumor deposits of >2 mm. ITCs are categorized into pN0, and in breast cancer, micrometastasis are treated as pN0, because further axillary LN dissection is not recommended when micrometastasis is identified in sentinel LNs. The guideline was supported by a series of reports, including evaluation with the B-32 protocol by Weaver et al. (3). They examined ITC/micrometastasis on survival in 3,887 patients with breast cancer who randomly assigned to sentinel LN biopsy plus axillary dissection or sentinel LN biopsy alone. Although outcomes of the patients with and without the metastases were statistically significant, the difference was limited to be minimal; overall survival (OS) (94.6% vs. 95.8%), disease-free survival (DFS) (86.4% vs. 89.2%), and distant disease-free interval (89.7% vs. 92.5%). Thus, ITCs and micrometastasis are not currently considered to be an aggressive clinical phenotype. Actually, the association of Breast Surgery Consensus Statement also recommends that, with regards to subsequent axillary treatment, patients with ITCs or micrometastasis should be managed as per node negative disease.
In contrast, both ITCs and micrometastasis can have clinical significance in lung cancer. Several techniques have been tried to detect micrometastasis in LNs with advances of molecular biology. Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) has been shown to be more sensitive than IHC for the detection of micrometastasis in patients with breast cancer (4). In lung cancer cases, the standard technique to evaluate LN metastases is histopathologic analysis. However, the detection of lymph nodal micrometastasis detected by IHC methods has reported to have strong prognostic impact for completely resected NSCLC patients (5-7). RT-PCR of CEA, CAM5 and PLUNC (8) and CEA alone (9) can estimate the presence of micrometastasis in LNs as well as an indicator of poor prognosis resected early stage NSCLC patients. Given that the presence of tumor cells in circulating peripheral blood, such as peripheral blood circulating tumor cells and cell free DNA, correlates with the recurrence rate and prognosis in resected NSCLC patients, clinical oncologic significance of LN micrometastasis between early stage breast cancer and NSCLC may be quite different. These findings make many pathologists hesitate to make the diagnoses for the lung cancer, although pN1 (mi) is defined in the general rule. Actually, there is no description about pN1 (mi) and pN0(i+) in the TNM classification of NSCLC, which is sharply contrasted to those of breast cancer.
Micropapillary proliferation is a proliferation distinct from conventional papillary proliferation, which has vasculature axis in the core. The first description about this micropapillary proliferation is reported in ovarian cancer. Re-analyses of the non-invasive ovarian cancer, which developed peritoneal recurrence afterward, revealed that micropapillary component should be considered as invasive growth pattern (10). Subsequently, it was reported that bladder cancers with micropapillary pattern showed strong invasiveness (11), and similar findings were also reported in many organs. In the lung, Amin et al. also first reported the association between high degree of vascular invasion and multiple organ metastasis with micropapillary proliferation and this finding has been confirmed in subsequent reports (10,12,13). Furthermore, meta-analysis of 19,502 lung adenocarcinomas showed higher rate of lymphatic invasion, worse overall survival and disease-free survival in lung cancer with micropapillary component (14).
The concept of “spread through air spaces (STAS)” has been proposed as a type of lung adenocarcinoma invasion (15). STAS is characterized as tumor cells spreading in air spaces into the lung parenchyma adjacent to the edge of the tumor. STAS was reported to be a significant prognostic factor for distant and locoregional recurrence in patients undergoing limited resection. STAS as originally described as micropapillary subtypes (16) is more frequently identified in patients with micropapillary component and the presence in the surgical specimens particularly with limited resection, is clinically critical. Frequent micropapillary structure of STAS might represent the aggressive nature as invasion to alveolar spaces. It is of note that micropapillary structure is always overrepresented in pleural effusion (17) (Figure 1).
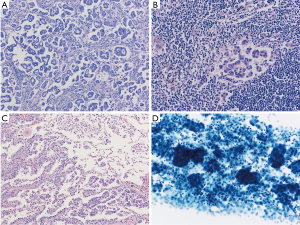
In conclusion, not like breast cancer, clinical significance of LN micrometastasis in lung cancer could not be neglectable, suggesting that application of TNM general rule on small sized metastasis is a matter of discussion. In contrast, aggressive nature of micropapillary component is widely recognized throughout the organs, suggesting that micropapillary formation is biologically associated with aggressive features independent of cancer origins.
Acknowledgements
Funding: The work is supported partly by the Grant-in-Aid for Scientific Research (B-16H05167).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6:660-2. [PubMed]
- Dai C, Xie H, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. [Crossref] [PubMed]
- Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med 2011;364:412-21. [Crossref] [PubMed]
- Noguchi S, Aihara T, Nakamori S, et al. The detection of breast carcinoma micrometastasis in axillary lymph nodes by means of reverse transcriptase-polymerase chain reaction. Cancer 1994;74:1595-600. [Crossref] [PubMed]
- Wu J, Ohta Y, Minato H, et al. Nodal occult metastasis in patients with peripheral lung adenocarcinoma of 2.0 cm or less in diameter. Ann Thorac Surg 2001;71:1772-7; discussion 1777-8.
- Osaki T, Oyama T, Gu CD, et al. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected stage I non-small-cell lung cancer. J Clin Oncol 2002;20:2930-6. [Crossref] [PubMed]
- Rena O, Carsana L, Cristina S, et al. Lymph node isolated tumor cells and micrometastasis in pathological stage I non-small cell lung cancer: prognostic significance. Eur J Cardiothorac Surg 2007;32:863-7. [Crossref] [PubMed]
- Benlloch S, Galbis-Caravajal JM, Alenda C, et al. Expression of molecular markers in mediastinal nodes from resected stage I non-small-cell lung cancer (NSCLC): prognostic impact and potential role as markers of occult micrometastasis. Ann Oncol 2009;20:91-7. [Crossref] [PubMed]
- Martin LW, D'Cunha J, Wang X, et al. Detection of Occult Micrometastasis in Patients With Clinical Stage I Non-Small-Cell Lung Cancer: A Prospective Analysis of Mature Results of CALGB 9761 (Alliance). J Clin Oncol 2016;34:1484-91. [Crossref] [PubMed]
- Hoshi R, Tsuzuku M, Horai T, et al. Micropapillary clusters in early-stage lung adenocarcinomas: a distinct cytologic sign of significantly poor prognosis. Cancer 2004;102:81-6. [Crossref] [PubMed]
- Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994;18:1224-32. [Crossref] [PubMed]
- Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 2004;45:580-6. [Crossref] [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [Crossref] [PubMed]
- Pyo JS, Kim JH. Clinicopathological Significance of Micropapillary Pattern in Lung Adenocarcinoma. Pathol Oncol Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Rodriguez EF, Shabihkhani M, Carter J, et al. Molecular Alterations in Patients with Pulmonary Adenocarcinoma Presenting with Malignant Pleural Effusion at the First Diagnosis. Acta Cytol 2017;61:214-22. [Crossref] [PubMed]


