High-amplitude left ventricular pacing in cardiac resynchronization therapy: an alternative way to increase response rate in non-responders
Introduction
Heart failure (HF) is a chronic progressive disease that affects a large part of the population, particularly the elderly, and it is associated with increased morbidity and mortality (1). Developments in drug therapy play a significant role in decreasing the mortality of HF. However, benefits of drug therapy are limited, and other treatment methods, such as cardiac resynchronization therapy (CRT), have been researched for HF patients resistant to drug therapy. CRT is commonly used to improve the survival rate of patients who have symptomatic HF and intraventricular conduction delay, despite medical therapy (QRS ≥120 ms).
In patients with moderate and advanced HF, CRT improves symptoms, capacity of exercise, and ventricular functions, and reduces the frequency of hospitalization and mortality. However, response to CRT differs from individual to individual. Approximately, 20-30% of patients who undergo CRT do not response to the treatment during clinical follow-up (2-4).
High-amplitude LV pulse is considered to provide faster conduction in patients undergoing CRT, and the myocardium cells in a wider area can be simultaneously stimulated as a result of this (5,6). We planned this study on the basis of the hypothesis that high-amplitude LV pacing would further reduce electrical and mechanical dyssynchrony, and positively affect LV systolic and functions diastolic.
Thus, here, we compare the effects of standard LV pacing amplitude with those of high LV pacing amplitude in order to improve the response rate to CRT, which is a proven effective treatment for HF.
Methods
This randomised controlled study was performed at 900-bed university hospital.
Patient selection
We initially included 46 CRT patients with ejection fraction (EF) ≤35%, QRS time ≥120 ms, and NYHA class III/IV symptoms of HF, despite optimal medical treatment. Of these patients, seven were lost to CRT follow-up, four due to high LV threshold, three due to lead dislocation. All patients were physically examined in detail; their height and weight were recorded, and they underwent standard 12-derivation electrocardiography. The patients were evaluated clinically and echocardiographically before, three and six months after CRT, and their pacemaker was adjusted. LV pulse amplitude of all patients was initially kept high (≥5 V). At the 3rd month, randomly selected 16 patients LV pulse amplitude (≥5 V) was not changed [high-amplitude group (HAG)].
The LV pulse amplitude of other 16 patients, who had a suitable threshold, was reduced at least twice the threshold (≤2.5 V) [low-amplitude group (LAG)]. Patients who had a survival time of less than six months, a severe psychiatric disorder, and who did not consent to this treatment were excluded from the study.
Implantation of the pacemaker
The CRT system was implanted in a standard fashion, CRT-D was implanted in 14 patients (87.5%) in HAG and ten patients (62.5%) in LAG. Implantations were performed to the rest of the patients in both groups. After implantation, right atrium (RA), RV, and LV amplitudes were adjusted to 3.5, 3.5, and 5.0 V, respectively.
Echocardiography
All patients underwent a transthoracic echocardiography in the 3rd and 6th month before and after CRT. Echocardiography was performed by two cardiologists who were blinded to the clinical data (FA and HZ). Parasternal long and short axes, apical two and four chamber echocardiographic views were obtained using the 2.5-3.5 MHz transducer and Vingmed system Vivid-5 (GE Ultrasound; Horten, Norway) echocardiography device, while echocardiographic examination was performed in the left lateral decubitus position. Evaluations were made using M-mode, two-dimensional, pulse wave (PW) Doppler, color Doppler, and PW tissue Doppler echocardiography. LV end-diastolic and end-systolic diameters were recorded using M-mode echocardiography on the parasternal long axis at the mitral chordal level. Left atrium anteroposterior diameter measured on the parasternal long axis was obtained using two-dimensional echocardiography. Endocardial borders were drawn on end-diastole and end-systole images with apical four chamber echocardiographic views, and end-diastolic and end-systolic volumes and EF were calculated by modified Simpson method (7).
Left ventricular filling time (LVFT) was measured using transmitral Doppler echocardiography. The ratio of LVFT to cardiac cycle length (RR) ≤40% was defined as atrioventricular dyssynchrony (8). The period between contractions of the septum and the posterior wall was calculated as septum-posterior wall motion delay (SPWMD) at the papillary muscular level on the parasternal short and long axes (9). This period being ≥130 ms was considered an indication of intraventricular dyssynchrony. The periods between QRS onset on pulse wave Doppler echocardiography at the aortic and pulmonary valve levels and the ejection onset were determined to be aortic and pulmonary pre-ejection time, respectively. The period between aortic pre-ejection time and pulmonary pre-ejection time was defined as interventricular motion delay (IVMD). This period being ≥40 ms was considered an indication of intraventricular dyssynchrony (10,11). Using PW tissue doppler echocardiography, the sample volume was placed in the basal portions of the septal and lateral walls (using the 4-chamber images), to derive velocity profiles. Timing of peak systolic velocity was measured from the onset of the QRS complex to the peak systolic velocity and expressed in milliseconds. A delay of ≥65 ms between the timing of the peak systolic velocities of the septum and the lateral wall was considered as intraventricular dyssynchrony (12).
Myocardial performance index (MPI) was defined as the sum of isovolumic contraction time (IVCT) and isovolumic relaxation time (IVRT) divided by the ejection time (ET) [MPI = (IVRT + IVCT)/ET]. MPI >0.5 was considered to be abnormal. MPI was calculated for both the left ventricle and right ventricle. The normal values are 0.28±0.04 and an increase was considered abnormal (13-15). Mitral regurgitation (MR) was evaluated qualitatively using color Doppler echocardiography and MR jet length. Systolic pulmonary artery pressure was estimated using tricuspid regurgitation jet velocity (TRV) based on the simplified Bernoulli’s equation [4× (TRV)2 + RA pressure] (16,17).
Response definition
Decrease in NHYA class by one or more in the sixth month was defined as clinical response, and decrease in the LV end-systolic volume (ESV) by ≥15% was defined as echocardiographic response.
Statistical analyses
Compliance of data with the normal distribution was assessed by Shapiro-Wilk normal distribution test. The numerical values with normal distribution are presented as mean ± standard deviation, and those with non-normal distribution are presented as median (minimum-maximum). Mann-Whitney U and t-tests were used for comparison of the groups. For repeated measures, ANOVA (repeated measures with ANOVA or paired sample t-test) and Wilcoxon tests were used to compare variables in different periods between the groups. Multivariate analysis was used to assess the response predictability of dyssynchrony parameters (SPWMD, IVMD, PW tissue doppler septal-lateral, and LVFT/RR) to CRT between groups. Chi-square test was used to assess whether there was a difference in the cause of HF and the accompanying diseases between the groups. SPSS 15.0 statistical software was used for assessment of data, and P<0.05 was considered statistically significant.
Results
The basic characteristics of the patients, such as age, gender, height, and weight, were similar between the two groups. In both groups, 12 patients had normal sinus rhythm and four had atrial fibrillation (AF). No significant differences were found between HAG and LAG in terms of prevalence of chronic renal failure (12.5% vs. 12.5%, P>0.05) and chronic obstructive pulmonary disease (COPD) (12.5% vs. 0%, P>0.05). Table 1 summarizes the basic characteristics of the patients included in the study.
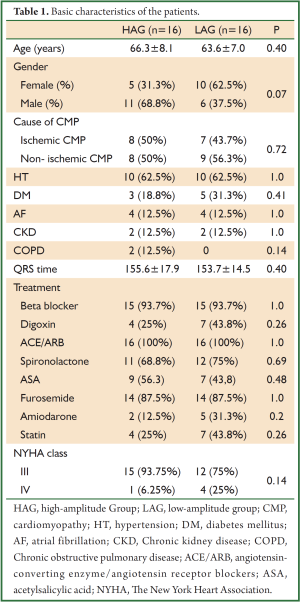
Full Table
Basic echocardiography parameters of both groups were similar in the assessment made prior to CRT (Table 2).
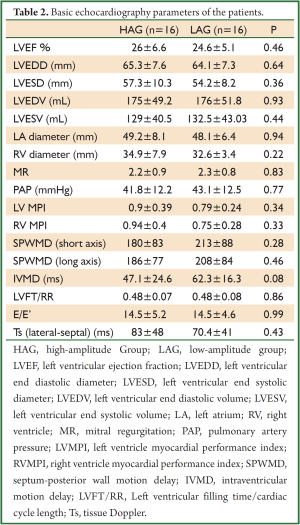
Full Table
Basic pace parameters recorded during the implantation of biventricular pacemaker were similar in HAG and in LAG. Parameters of the pacemaker are shown in the Table 3.
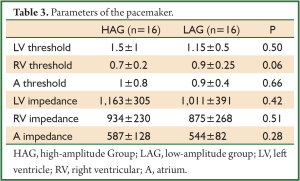
Full Table
Onset within and between the groups and echocardiographic parameters in the 3rd and 6th month are presented in Table 4. On comparing the two groups within the same periods (baseline, 3rd month, and 6th month), no statistically significant differences were found in the values of LV EF, LV ESV, LV EDV, PAP, LV MPI, and RV MPI.
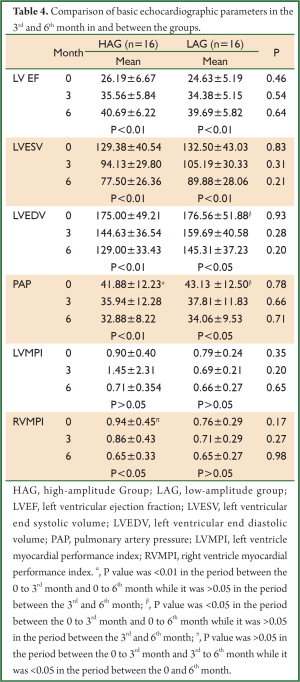
Full Table
On comparing the parameter values of different periods within the same group, increase in LV EF and decrease in LV ESV at baseline, 3rd month, and 6th month were found to be statistically significant in both groups (P<0.01). Decrease in EDV was significant in HAG in the periods between baseline, 3rd month, and 6th month (P<0.01). Decrease in EDV was statistically significant in LAG in the 3rd and 6th month compared to onset (P<0.05); however, decrease in the same parameter in the period between the 3rd and 6th month was not statistically significant (P>0.05) (Figure 1).
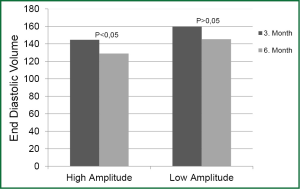
Decrease in PAP in the 3rd and 6th month was significant in both groups compared to onset (P<0.01 and P<0.05, respectively). Decrease in the period between the 3rd and 6th month was not statistically significant in either group.
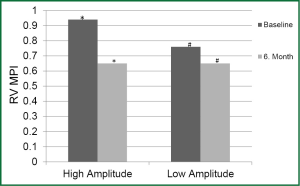
Difference in LV MPI between the periods was not significant in both groups. Decrease in RV MPI in the 6th month was significant in HAG compared to the onset (P<0.05); however was not significant in LAG (Figure 2).
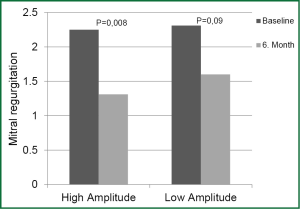
Decrease in MR in HAG was statistically significant in the 6th month compared to onset while decrease in MR in LAG was not statistically significant in the same period (P=0.008 and P=0.09, respectively) (Figure 3).
In the correlation analysis, performed using the data of both groups, statistically significant positive correlation was observed in terms of percentage change in ESV in the 3rd and 6th month before and after CRT, along with an improvement in NYHA functional class. The lower the EF, the higher the LV ESV and LV EDV at onset; increase in EF had a statistically significant correlation in the 3rd and 6th month after CRT.
With respect to changes in EF and ESV as echocardiographic response criteria of the patients to CRT, none of the variables of dyssynchrony parameters, including SPWMD (short and long axes), IVMD, LVFT/RR were statistically significant in predicting the response to CRT, according to multivariate analysis. On the other hand, the length of SPWMD (long axis) was found to be a better predictor of the percentage change in EF compared to other parameters (P=0.066).
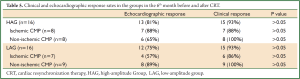
No significant differences were found between HAG and LAG on assessment of patients for clinical and echocardiographic response in the 6th month before and after CRT. In addition, no significant differences were found in both groups when ischemic patients were compared with non- ischemic patients (Table 5).
Full Table
Discussion
In this study, we demonstrate that CRT performed with high-amplitude LV pacing gave more favorable values of clinical and echocardiographic parameters than did CRT performed with standard LV pacing. This is the first study evaluated the effect of high amplitude LV pacing on end points.
CRT has been shown to improve quality of life, exercise performance, and LVEF in NYHA class III and IV HF patients with intraventricular conduction defect, and reduce mortality and hospitalization associated with HF (4,18,19). However, approximately 20-30% of patients receiving CRT do not benefit from this therapy clinically (3,4).
This study was based on the notion that in patients undergoing CRT, LV pacing treatment would simultaneously stimulate the myocardium cells in a wider area through high pulse amplitude and provide faster conduction, and consequently, electrical and mechanical dyssynchrony would reduce further, resulting in a more positive effect on LV functions. Using this hypothesis, treatments using high- and low-amplitude LV pacing were compared and differences in clinical and echocardiographic responses of patients to CRT were studied.
In CARE-HF and REVERSE studies, which consider the effects of CRT over remodeling of LV, significant improvements in LV size and function, LVEF, RV function, LV size, and severity of MR were seen in the patients who received CRT compared to those who underwent ICD implantation alone (20,21). In our study, an increase in LV EF and a significant decrease in LV ESV, LV EDV, MR, and PAP were observed in both groups.
Our study is the first to assess the effect of high pulse amplitude and LV pacing over the response to CRT. Sauer et al. (5) showed in one of their studies that high LV pacemaker output stimulated a greater myocardial area to obtain a faster conduction. The study was performed on 42 patients who had received CRT, and examination of the RV electrogram of these patients revealed a significant decrease in interventricular conduction time. However, how this affected synchronization was not evaluated. In our study, we aimed to evaluate the contribution of this effect to the clinical and echocardiographic response. No statistically significant differences were noted in the increase in EF and the decrease in ESV between HAG and LAG patients. While the decrease in EDV was statistically significant in HAG between the 3rd and the 6th month, it was not statistically significant in LAG during the same period. EDV continued to reduce even after the 3rd month in HAG, but the decrease was not significant in LAG where pulse amplitude was reduced. The decrease in EDV in LAG patients after the 3rd month was not due to the decreased LV pulse amplitude. These improvements in LV functions of HAG patients was considered to be a positive effect of high-amplitude pace.
In this study clinical and echocardiographic response was similar in two groups. This may be related with small size of cohort and short follow up period.
MPI is an important parameter that indicates the systolic and diastolic functions of both the RV and LV. The MPI was significantly higher in the patient group in a study where patients with idiopathic dilated cardiomyopathy and normal subjects were investigated. The same study also reported that the most important determinants of the endpoints of cardiac transplantation and cardiac- or non-cardiac-related mortality of unknown cause during the 5-year follow-up were MPI and LV EF (22). In another study, the mean MPI value was 1.06 in patients with severe dilated cardiomyopathy, 0.59 in patients with moderate dilated cardiomyopathy, and 0.39 in normal subjects, indicating a significant difference between the groups (23). MPI appears to be a useful parameter for the follow-up of patients with HF. In our study, decrease in RV MPI in the 6th month was statistically significant in HAG patients, while no significant decrease in LAG patients was observed. This decrease in MPI in HAG patients might have arisen from an indirect positive effect of high-amplitude LV pacing over RV systolic functions. On the other hand, one study reported that CRT not only stimulates reverse LV remodeling but also results in significant RV reverse remodeling (24). Lower RV MPI in HAG patients suggests that the effect of high-amplitude pacing is more significant on RV reverse remodeling.
Functional MR is very common in patients with HF. Moderate advanced MR is responsible for the progression of HF, aggravation of symptoms, and adverse events. CRT has a potential to reverse the degree of MR (25). In our study, decrease in MR at the six-month follow-up was statistically significant in HAG patients, not in LAG patients. Absence of the positive effects of high-amplitude LV pacing in LAG patients supports our hypothesis that high LV pacing could be more effective in improving patients’ response to CRT.
The response rates of patients with ischemic and non-ischemic cardiomyopathy to CRT are different. In the study by Sauer et al., the decrease in the interventricular conduction time in patients with ischemic cardiomyopathy was found to be statistically significant compared to the decrease in patients with non-ischemic cardiomyopathy (5). This difference may be due to the presence of more scar tissues in the area where the LV lead is placed in the patients with ischemic cardiomyopathy, and due to a change in the LV depolarization time caused by stimulating the high LV pulse amplitude (5). In the studies by MUSTIC and MIRACLE, improvement in LV systolic functions was more significant in patients with non-ischemic cardiomyopathy compared to patients with ischemic cardiomyopathy (26,27). Similarly, in the subgroup analysis of the PROSPECT study, clinical and echocardiographic response rates were found to be higher in the non-ischemic cardiomyopathy group than in the ischemic group (28). This result is probably associated with the presence of a considerable amount of scar tissue, which limits LV remodeling in patients with ischemic cardiomyopathy (29-31). Considering the etiologic characteristics of the patients in our study, 50% of the patients in HAG and 56.3% in LAG were non-ischemic. In our study, when we compared patients with ischemic etiology, who were expected to exhibit better synchronization following stimulation by high LV pulse amplitude, with patients with non-ischemic etiology for echocardiographic response, the response rates of the former was not statistically significant yet it was quantitatively higher. It may be related with small sample sizes and short follow-up periods. No statistically significant difference was observed between patients with ischemic and non-ischemic etiology in the LAG for clinical and echocardiographic response.
The life of the pacemaker battery is significantly reduced when pulse amplitude value is adjusted to >2.5 V (32). Therefore, setting a high voltage for each patient may require frequent replacement of the battery. This is disadvantageous as it bears an additional cost and involves more work. Thus, high-amplitude LV pacing should be considered only for patients non-responsive to conventional CRT.
In order to assess the effects of high-amplitude LV pacing on both patients with ischemic and non-ischemic cardiomyopathy, a wider range of randomized controlled studies with long-term follow up need to be assessed.
Limitations
This study had three limitations. Firstly, this study was conducted only on a small size of CRT patients. Secondly, we only followed these patients for a period of three months with different pacing outputs, which may not be enough time to demonstrate a difference. Thirdly, electrical activation patterns not been assessed between the LAG and HAG might this have provided additional proof that high output pacing recruits more myocardium, thus supporting the hypotheisis.
Conclusions
The present study is the first to assess the clinical and echocardiographic effects of a biventricular pacemaker administrated with high LV pulse amplitude. In this prospective study, we shown that CRT performed with high-amplitude LV pacing gave more favorable values of clinical and echocardiographic parameters than did CRT performed with standard LV pacing. In light of these findings, LV pacing with high-amplitude appears to be useful at least for patients non-responsive to conventional CRT.
Acknowledgments
This project was supported by Ondokuz Mayis University (PYO.TIP.1904.10.005).
Disclosure: The authors declare no conflict of interest.
References
- Onwuanyi A, Taylor M. Acute decompensated heart failure: pathophysiology and treatment. Am J Cardiol 2007;99: 25D-30D. [PubMed]
- Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873-80. [PubMed]
- Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53. [PubMed]
- Sauer WH, Bristow MR. The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial in perspective. J Interv Card Electrophysiol 2008;21:3-11. [PubMed]
- Sauer WH, Sussman JS, Verdino RJ, et al. Increasing left ventricular pacing output decreases interventricular conduction time in patients with biventricular pacing systems. Pacing Clin Electrophysiol 2006;29:569-73. [PubMed]
- Theis C, Bavikati VV, Langberg JJ, et al. The relationship of bipolar left ventricular pacing stimulus intensity to cardiac depolarization and repolarization in humans with cardiac resynchronization devices. J Cardiovasc Electrophysiol 2009;20:645-9. [PubMed]
- Otto CM. Left and right ventricular systolic function. In Otto CM. eds. Textbook of Clinical Echocardiography. 3rd edition. Philadelphia: WB Saunders, 2004:131-65.
- van Bommel RJ, Bax JJ, Abraham WT, et al. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J 2009;30:2470-7. [PubMed]
- Pitzalis MV, Iacoviello M, Romito R, et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 2002;40:1615-22. [PubMed]
- Bax JJ, Ansalone G, Breithardt OA, et al. Echocardiographic evaluation of cardiac resynchronization therapy: ready for routine clinical use? A critical appraisal. J Am Coll Cardiol 2004;44:1-9. [PubMed]
- Cazeau S, Bordachar P, Jauvert G, et al. Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol 2003;26:137-43. [PubMed]
- Bax JJ, Molhoek SG, van Erven L, et al. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2003;91:94-7. [PubMed]
- Feigenbaum H, Armstrong WF, Ryan T. Feigenbaum’s Echocardiography. 6th edition. Philadelphia: Lippincott Williams & Wilkins, 2005:138-69, 437-86.
- Vuille C, Weyman AE. Left ventricle 1. General considerations assesment of champer size and function. In Weyman AE. eds. Principles and practise of Echocardiography. Second edition. Philadelphia: Lea & Febiger, 1994:575-625.
- Reynolds T. The Echocardiographer’s Pocket Reference. 2nd ed. USA: Arizona Heart Institute, 2000:216.
- Chan KL, Currie PJ, Seward JB, et al. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol 1987;9:549-54. [PubMed]
- Feigenbaum H, Armstrong WF, Ryan T. Tricuspid and pulmoner valves. In: Feigenbaum H. eds. Feigenbaum’s Echocardiography. 6th edition. Philadelphia: Lippincott Williams & Wilkins, 2005;362.
- McAlister FA, Ezekowitz JA, Wiebe N, et al. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med 2004;141:381-90. [PubMed]
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49. [PubMed]
- Cleland JG, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase. Eur Heart J 2006;27:1928-32. [PubMed]
- Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834-43. [PubMed]
- Dujardin KS, Tei C, Yeo TC, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 1998;82:1071-6. [PubMed]
- Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol 1995;26:357-66. [PubMed]
- Bleeker GB, Schalij MJ, Nihoyannopoulos P, et al. Left venricular dyssynchrony remodeling after cardiac resynchronization therapy. Journal of the American College of Cardiology 2005;46:2264-9. [PubMed]
- Di Biase L, Auricchio A, Mohanty P, et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace 2011;13:829-38. [PubMed]
- Duncan A, Wait D, Gibson D, et al. Left ventricular remodelling and haemodynamic effects of multisite biventricular pacing in patients with left ventricular systolic dysfunction and activation disturbances in sinus rhythm: sub-study of the MUSTIC(Multisite Stimulation in Cardiomyopathies). Eur Heart J 2003;24:430-41. [PubMed]
- Sutton MG, Plappert T, Hilpisch KE, et al. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Circulation 2006;113:266-72. [PubMed]
- Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008;117:2608-16. [PubMed]
- Chalil S, Foley PW, Muyhaldeen SA, et al. Late Gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace 2007;9:1031-7. [PubMed]
- White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 2006;48:1953-60. [PubMed]
- Ypenburg C, Roes SD, Bleeker GB, et al. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol 2007;99:657-60. [PubMed]
- Kindermann M, Kusch O, Fröhlig G, et al. Safety and efficiency of pulse charge multiplication for chronic ventricular output programming. Pacing Clin Electrophysiol 2001;24:430-40. [PubMed]


