Lentivirus vector-mediated Rho guanine nucleotide dissociation inhibitor 2 overexpression induces beta-2 adrenergic receptor desensitization in airway smooth muscle cells
Background
Beta-2 adrenergic receptor (β2AR) gene polymorphisms are critical to the response of asthma patients to both short- and long-acting β2AR agonists, the most effective class of bronchodilators and a mainstay of asthma management (1). Worldwide prevalence of adult asthma has been reported at 4.3%, though more than 21-times this number have been reported in some countries and increasing trends have been reported with urbanization (2). In asthma patients, β2AR downregulation is critical to the action of asthma rescue treatments based on β2AR agonists (3). However, multi-mechanism hormonal desensitization can result in tolerance, or bronchodilator desensitization, leading to rescue treatment failure (3). Thus, there is an urgent need to better understand the complex mechanisms involved in bronchodilator desensitization in order to develop preventative strategies.
Short-acting β2AR agonists are life-saving rescue agents, though regular use is not recommended due to safety and tolerance-development concerns (4). Similar concerns pertaining to long-acting β2AR agonists have restricted these medications to patients with asthma that is not acceptably controlled by inhaled corticosteroids (5). β2AR agonists predominantly function through β2AR downregulation, characterized by the sustained activation of G protein (guanylate binding protein)-coupled receptors, thus resulting in a time-dependent reduction of receptor density in intact cells (3). Frequent β2AR downregulation, however, may contribute to β2AR desensitization through mechanisms such as receptor phosphorylation, G-protein uncoupling, receptor internalization, decreased receptor mRNA and protein synthesis, and increased receptor degradation (6).
The mechanistic process of β2AR desensitization involves initial rapid desensitization followed by heterologous and homologous desensitization (6). Homologous desensitization is mediated by G protein-coupled receptor kinases (GRKs) with specific roles in ligand binding (7). Furthermore, considerable evidence indicates that carboxyl-terminal serine 355, 356, and 364 may play roles in GRK-mediated phosphorylation, thus contributing to β2AR desensitization (7,8). Most contemporary research agrees that G protein-coupled receptor kinase 2 (GRK2) is responsible the majority of agonist-dependent receptor phosphorylation, central to β2AR desensitization (9).
Agonist stimulation of the β2ARs has been reported to lead to both ubiquitination and lysosomal degradation, central features of β2AR desensitization (10). In asthmatic and β2AR desensitization mouse models, the levels of inflammatory cells, bronchoalveolar lavage fluid (BALF) cytokines, serum immunoglobulin E (IgE), and, most notably, the protein target of ubiquitin, Rho guanine nucleotide dissociation inhibitor 2 (RhoGDI2), have been reported to be statistically increased in mice exhibiting β2AR desensitization in recent comparative proteomics studies (11-13). RhoGDI2 is an important regulating factor of Rho (Ras homologue). Additionally, lysosomal degradation leading to progressive β2AR desensitization is regulated by Rab11, which impacts recycling and lysosome targeting of β2-adrenergic receptors, and Rab11 binding is determined, in part, by Arg(333) and Lys(348) (14). Because both ubiquitination and lysosomal degradation are central mechanisms in the development of β2AR desensitization (10), the role of RhoGDI2 in β2AR desensitization merits further exploration. As the regulatory mechanism we determine to study which is relevant to Rho, guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) are both regulatory factor of Rho, so RhoGEF and RhoGAP may play an important part in the process. RhoGEF can increase the activity of Rho protein and RhoGAP down-regulates activity of Rho protein.
The current study employs airway smooth muscle cells (ASMCs) from mice transfected with lentivirus vector-mediated RhoGDI2 overexpression to explore the effect of RhoGDI2 overexpression on β2AR desensitization. Furthermore, mRNA and protein expressions of RhoGDI2, β2AR, GEF, GAP, and GRK2 were explored to provide a basis for determination of the relationship between these proteins and β2AR desensitization.
Materials and methods
Animal subjects
Female BALB/c mice aged 6-8 weeks (mean body weight 20±2 g) were purchased from the Laboratory Animal Center of Nantong University (Nantong, China). All mice were housed in temperature-controlled cages at 20-25 °C with a 12-hour dark/light cycle and with ad libitum access to food and water for one week prior to the experiments. BALB/c mice ASMCs from mice were grouped as untreated (control), GFP lentivirus-infected (negative control), and RhoGDI2 vector-infected (RhoGDI2 overexpression group) for these experiments. All animal protocols were approved by the Institutional Animal Care and Use Committee of Nantong University and conformed to the International Guidelines for Ethical Use of Animals.
Primary culture and purification of ASMCs
Mice ASMCs were primarily and serially cultured. ASMCs were purified by differential adherence (15) and identified by appraisal α-actin protein immunocytochemical staining, as previously described (16,17). Briefly, the trachea and pulmonary tissues were collected from mice killed by neck-breaking and soaked in 75% alcohol for 2 min. Hyaline tissues were attained by removing tracheal ectoblasts, cutting the trachea lengthways with ophthalmic tweezers, and scraping the inner membrane gently with scalper. Resultant hyaline tissues (<1 mm3) were affixed equidistantly with dental probe in the bottom of a 25 mL culture bottle. Cells were cultured in covered, inverted culture bottles with 2 mL high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, USA) containing 20% fetal bovine serum (FBS) (GIBCO, USA) at 37 °C, in a 5% CO2 incubator for 3 hours without agitation, in which cells did not touch medium. Upon attaining dry tissues, bottles were inverted to submerge cells in the medium, cultured for 3 days with loose covering, and mixed with 5 mL of new medium on day 3. All medium was replaced on day 6, and thereafter half the medium was changed every 5 days. When outer cell boundaries were fused >80% under inverted phase contrast microscope (Olympus, Tokyo, Japan), medium was removed, cells were washed D-Hanks solution 3×, 2 mL of 0.25% trypsin was added with mild agitation to ensure even distribution, and cells were cultured for 5 min, producing bright appearance and retracted cytoplasm under microscopic examination. Bottles were briefly agitated, and 6 mL of high-glucose DMEM containing 20% FBS was added to stop digestion. Cells were dried repeatedly with TubularisTM (Biomics biotechnologies, China) and then inoculated at 1:1, 1:2, or 1:3 densities. Half of the medium was changed at 5-day intervals.
For purification, cells were digested with 0.25% trypsin, and DMEM containing 20% FBS was added for 15 min. Non-adherent cells were separated and subcultured for 15 min, which was repeated for twice, thereby using the smaller adherence time of fibroblasts (10-30 min adherence) compared to smooth muscle cells (1-4 h adherence) to separate and purify ASMCs (15).
Immunocytochemical staining
SABC-FITC immunocytochemical staining was conducted using an immunohistochemical kit (Wuhan Boster Biotechnology Ltd., China), according to the instructions provided by the manufacturer. Briefly, cells cultured to 80% fusion were fixed with 4% paraformaldehyde for 20 min, washed with distilled water 3× for 3 min, subjected to 0.6% hydrogen peroxide for 30 min and 0.01 mol/L phosphate buffered saline (PBS) solution 3× for 2 min, treated with normal goat serum at room temperature for 20 min without washing, and treated with mouse Anti-α-actin monoclonal antibody (Wuhan Boster Biotechnology Ltd., China) for 20 min. Slides were then washed with 0.01 mol/L PBS 3× for 2 min, treated with biotinylation Goat Anti-Rabbit IgG (Santa cruz biotechnology, USA) for 20 min, washed with 0.01 mol/L PBS 3× for 2 min, treated with SABC-FITC for 20 min, and washed with 0.01 mol/L PBS for 3× for 5 min. Cells were then observed and photographed under a laser scanning microscope (Olympus, Tokyo, Japan).
Production of RhoGDI2 recombinant lentivirus vectors
The RhoGDI2 gene (NCBI NM_001175.4) coding sequence was amplified by PCR and subcloned into a lentivirus expression plasmid pWPXL-eGFP vector (TronoLab, France) along with BamH I and Mlu I restriction sites to construct a lentivirus-based overexpression vector carrying the RhoGDI2 sequence (pWPXL -eGFP-RhoGDI2), confirmed by PCR and DNA sequencing. The lentivirus expression plasmid pWPXL-eGFP-RhoGDI2 and two packaging plasmids psPAX2 and pMD2.G (TronoLab, France) were co-transfected into human embryonic kidney cells (HEK293T) in serum-free medium using LipofectamineTM 2000 (Invitrogen Co., Carlsbad, CA), according the manufacturer’s instructions. Additionally, a lentivirus expression plasmid without RhoGDI2 gene and two packaging plasmids were co-transfected into 293T cells to construct a blank control lentivirus. At 8 h after transfection, medium was completed replaced. At 48 h after transfection, culture medium was collected and centrifuged at 4,000 ×g and 4 °C for 10 min. The supernatant was filtered (0.45 μm) into a Plus-20 centrifugal ultrafiltration (Biomics biotechnologies, China) and centrifuged at 4,000 ×g to obtain high-titer lentivirus.
Establishment of stable RhoGDI2 overexpression ASMCs
Primary ASMC cultures were plated at a concentration of 1.5×105 cells/well in six-well plates and infected with RhoGDI2-GFP lentivirus vectors (RhoGDI2 overexpression group), GFP lentivirus vectors (negative control group), or no infection (control) in serum-free medium for 12 h. Cells were then washed and embedded in complete medium for 3 days. GFP expression was examined by fluorescence microscopy (Olympus, Tokyo, Japan). 4 days after infection, ASMCs were used to the subsequent experiments.
Real-time RT-PCR detection of RhoGDI2, β2AR, GEF, GAP, and GRK2 mRNA expressions
Total RNA was extracted from ASMCs with Trizol reagent (Invitrogen, USA). The ratio of absorbance at 260 and 280 nm (A260/280) was used to assess RNA purity. cDNA synthesis was performed using oligo (dT) primer (Sangon BiotechCo., Ltd, Shanghai, China) and M-MLV reverse transcriptase (Promega, USA). Quantitative real-time PCR detection was performed using a SYBR green real-time PCR kit (Takara, Japan) in real-time PCR system (BioRad, USA). Housekeeping gene α-tubulin expression was quantified and used for normalization. All primers were designed using Primer Premier 5 (PREMIER Biosoft International, Palo Alto, California, USA) (Table 1). Thermal cycling was conducted for 5 min at 95 °C (pre-denaturation), followed by 25 cycles at 95 °C for 20 s (denaturation), 55 °C for 30 s (annealing), and 72 °C for 30 s (stretching), and 72 °C for 7 min (final stretching). 2–ΔΔCT was used to calculate relative changes in gene expression from qRT-PCR (ΔCT = CTtarget gene–CTα-tubulin, ΔΔCT = ΔCT experimental –ΔCT control).
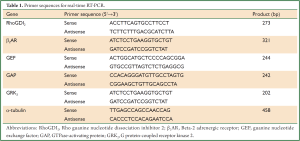
Full Table
Western blot detection of RhoGDI2, β2AR, GEF, GAP, and GRK2 protein expressions
ASMCs were washed 2× with cold PBS, treated with 2× lysis buffer comprised of 100 mmol/L Tris-HCl (pH=6.8), 20 g/L mercaptoethanol, 200 g/L glycerol, and 40 g/L SDS, and lysed on ice for 10 min. Cells were homogenized by ultrasonication, and centrifuged at 4 °C at 12,000 ×g for 15 min. Protein concentration was measured by trace protein nucleic acid analyzer (Implen, Germany). Protein samples (20 ug) were subjected to 8% SDS-PAGE and transferred onto poly (vinyl idene fluoride) (PVDF) membranes (Pall, USA) treated with tris-buffered saline and tween-20 solution (TBST) containing 50 g/L skim milk at room temperature for 1 h. Then, rabbit anti-mouse RhoGDI2 (FL-201) polyclonal antibody (sc-11359), rabbit anti-mouse β2AR (H-73) polyclonal antibody (sc-9042), rabbit anti-mouse RhoGEF [p115/Lsc (H-165)] polyclonal antibody (sc-20804), rabbit anti-mouse ARHGAP[22/24 (H-162)] polyclonal antibody (sc-99112), rabbit anti-mouse GRK2 (C-15) polyclonal antibody (sc-562), and rabbit anti-mouse a-tubulin polyclonal antibody (sc-135659) (Santa Cruz Biotechnology, CA, USA) were added and incubated at room temperature for 2 h. Membranes were washed 3× with TBST and incubated with diluted horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, CA, USA) at room temperature for 2 h. Membranes were washed 3× with TBST, and bands were detected using an ECL + plusTM kit (Beyotime Ltd., China) The relative protein expressions were normalized to a-tubulin.
Radioligand receptor binding assay
ASMCs were centrifuged at 250 ×g at 4 °C for 10 min, and the resultant pellet was resuspended in 8 volumes of hypotonic buffer at 0 °C, allowed to swell for 10 min, and centrifuged at 12,000 ×g at 4 °C for 15 min. When 90% of cells were broken, as determined by phase-contrast microscopy, the pellet was resuspended in binding buffer and homogenized with a Polytron homogenizer (Kinematica, Basel, Switzerland). ASMCs were incubated with (125I) iodocyanopindolol (ICYP) (sp act: 7,400×1,010 Bq/mmol; 3-100 pmol/L) (GE Healthcare Life Sciences, USA) in the presence or absence of excess Iso (Nuclear Medicine, Shanghai University of Traditional Chinese Medicine, China) (200 μmol/L) in 25 mmol/L Tris-HCl buffer (pH 7.4) containing 154 mmol/L NaCl and 1.1 mmol/L ascorbic acid to prevent oxidation of Iso, in a final volume of 250 μL. β1AR density was analyzed by ICYP saturation binding in the presence of 0.1 μmol/L ICI 118551 (Nuclear Medicine, Shanghai University of Traditional Chinese Medicine, China), a β2-selective antagonist. At this concentration, virtually all β2-receptors were occupied. β2AR density was analyzed by ICYP saturation binding in the presence of 0.1 μmol/L CGP 20712 A (Nuclear Medicine, Shanghai University of Traditional Chinese Medicine, China), a β1-selective antagonist (18). Cells were incubated in triplicate at 37 °C for 120 min for optimal specific binding, and terminated by rapid filtration through GF/C glass-fiber filters (Whatman Inc., Clifton, NJ, USA). Each filter was rapidly washed with 3×5 mL ice-cold 25 mmol/L Tris-HCl buffer (pH 7.4) and counted in the Auto Gamma Counting System (PerkinElmer, USA) at an efficiency of 80%. Specific binding was calculated by subtracting nonspecific binding from total binding. Protein concentration was determined by Lowry method with bovine serum albumin as a standard.
Statistical analysis
All data were reported as means ± standard deviation (SD), and statistical analyzed by SPSS vs.17.0 (SPSS, Inc, Chicago, USA). Normally distributed and homogenous data sets were compared by one way ANVOA and the least significant difference method (LSD) as post hoc test. P-values of less than 0.05 were considered statistically significant (P<0.05).
Results
ASMC growth
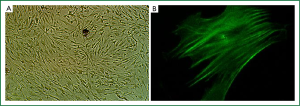
Primarily cultured ASMCs demonstrated typical characteristics, including a peak and valley growth trend (Figure 1A). Furthermore, specific smooth muscle α-actin filaments were observed parallel to the longitudinal axis in the cytoplasm (Figure 1B). The percentage of positive expression of specific smooth muscle α-actin filament was more than 95%.
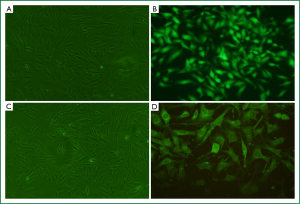
Stable overexpression of RhoGDI2 in ASMCs
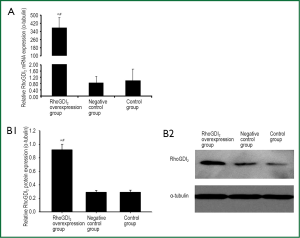
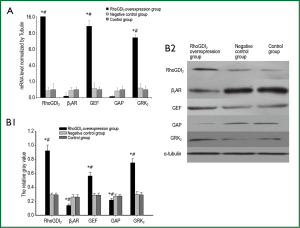
The recombinant lentivirus plasmid pWPXL-RhoGDI2 was successfully constructed, and stablely transfected into ASMCs in the RhoGDI2 overexpression group expressing GFP were observed under a fluorescence microscope (Figure 2). Fluorescence microscopy indicated infection efficiency >80%. Real-time RT-PCR demonstrated that the mRNA expression of RhoGDI2 in the RhoGDI2 overexpression group was about 377-fold higher than that in the control group (Figure 3A). Furthermore, western blot indicated that the protein expression of RhoGDI2 was significantly higher in the RhoGDI2 overexpression group than those in other groups (both P<0.05). The protein expression of RhoGDI2 in the RhoGDI2 overexpression group was approximately 3-fold higher than that observed in the control group (Figure 3B). There were no statistically significant difference in the mRNA and protein expressions of RhoGDI2 between the negative control group and the control group (both P>0.05).
The effects of RhoGDI2 overexpression on mRNA and protein expressions of β2AR, GEF, GAP and GRK2 in ASMCs
The mRNA expression of β2AR was significantly lower in the RhoGDI2 overexpression group than those in other groups (both P<0.05). The mRNA expressions of GEF and GRK2 were significantly increased along with increasing RhoGDI2 expression, demonstrating a positive relationship (all P<0.05). Conversely, the mRNA expression of GAP significantly decreased with increasing RhoGDI2, demonstrating a negative relationship (all P<0.05) (Figure 4A). Similar trends were observed in protein expressions, with RhoGDI2 overexpression associated with significantly increased GEF and GRK2 protein expressions while GAP and β2AR protein expressions were significantly decreased (all P<0.05) (Figure 4B).
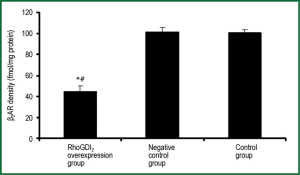
The effects of RhoGDI2 overexpression on β2AR density in ASMCs
β2AR density was significantly lower in the RhoGDI2 overexpression group than those in other groups (both P<0.05), with control and negative control groups exhibiting β2AR density more than 2-fold higher than that observed in the RhoGDI2 overexpression group (Figure 5). No significant difference was observed between the control group and negative control group in terms of β2AR density (P>0.05).
Discussion
In mouse ASMCs transfected with lentivirus vector-mediated overexpression of RhoGDI2, mRNA and protein expressions of RhoGDI2, GEF, and GRK2 were significantly increased while β2AR and GAP expression were significantly decreased. According to our experimental results, we draw the Schematic diagram for the regulation of β2AR by RhoGDI2 (Figure 6). Furthermore, β2AR density in RhoGDI2 overexpression group was less than half as high in the control group, indicating that RhoGDI2 may contribute to the reduced β2AR density observed in cellular β2AR desensitization. Thus, the role of RhoGDI2 in reducing β2AR density is an important contributing factor in the complex mechanism associated with bronchodilator tolerance in asthma patients, with these findings meriting further investigation in humans.
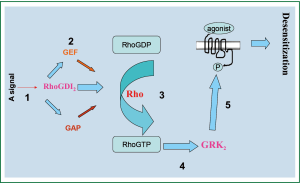
The mechanism of RhoGDIs action has been previously reported, with the molecule reported to act primarily as a down-regulator of Rho family GTPases and thus prevent nucleotide exchange and membrane association (19). Cytoplasmic RhoGDIs inactivate GTPases by combining with GDP Rho proteins, stranding them in the cytoplasm and inhibiting GDP dissociation from Rho proteins; furthermore RhoGDIs terminate signal transduction and regulate negative feedback (19). Recent evidence has also suggested that RhoGDIs may be positive regulators of Rho activities (20). Consist with previous findings that asthmatic mice exhibit β2AR desensitization verified by inflammatory cell count, cytokine concentration of BALF, and serum IgE level when exposed to overexpression of RhoGDI2 (11), the current study supports that RhoGDI2 influences β2AR desensitization. If RhoGDI2 plays a negative regulatory role in β2AR desensitization process, RhoGDI2 may hypothetically be able to inhibit Rho protein activity, though this hypothesis requires further exploration. In previous studies of heart failure, it has been reported that inhibition of GRK2 can attenuate β2AR desensitization (21). Furthermore, these findings in heart failure have been linked with G-protein β2AR signaling, resulting in maladaptive remodeling and treatment failure (22). Consistent with these findings, the current results indicate that as GRK2 decreases, β2AR also decreases, which may indicate that RhoGDI2 acts as a regulator of β2AR important in the β2AR desensitization process.
The current study indicates that RhoGDIs are important regulators of GEF and GAP activities. Previously, it has been reported that GEF can increase the activity of Rho protein, thus accelerating hydrolyzation of GTP in a similar manner as GRKs (23). Furthermore, RhoGEFs catalyzes the release of GDP and combined GTP, thereby activating the RhoGTP enzyme, and RhoGAPs down-regulate Rho protein activity, thereby increasing the activity of GTPase (23). Notably, the current RT-PCR and western blot studies consistently indicated that GAP expression decreased as RhoGDI2 increased, suggesting that RhoGDI2 may inhibit GAP activity. These findings are consistent with previous reports in metastatic cancer indicating that RhoGDI2 metastasis inhibition works through Rho GTPases and potentially influence GAP hydrolysis in a mechanism distinct from membrane association inhibition (24).
RhoGDI2 has been reported to mediate GRK2 through the Rho protein, but the effect RhoGDI2 has on GRK2 is remains uncharacterized (25). In previous studies in genetically modified mice, GRK2 has been linked with abnormal airway and cardiac responses (26), further indicating the potential importance of GRK2 during the treatment response of asthma patients. Notably, the expression of RhoGDI2 in the current study was found to positively correlate with that of GRK2. Thus, RhoGDI2 likely plays an important role in increasing the activity of GRK2, performing a positive role in β2AR sensitization; however, further studies of the exact, direct role of Rho will be required to fully determine this mechanistic pathway. Hypothetically, the action of RhoGDI2 through the phosphorylation of its acceptor (7-9) suggests that it may also play a role in upstream intra-cellular signaling pathways relevant to bronchodilator desensitization. However, it remains likely that other, yet undetermined, intimidate factors exist.
In the current study, only indirect comparison of RhoGDI2 and Rho expression using both mRNA and protein level are possible, a central limitation of the current experiments. Thus, further experiments will be required to fully elucidate the role of Rho in β2AR desensitization. The full utility of lentivirus-based vector systems as clinical alternatives has not been fully investigates, necessitating extensive further work before these results can be translated into meaningful clinical interventions for β2AR desensitization. Furthermore, the current data as well as data from subsequent in vivo and in vitro studies should be more carefully statistically analyzed by correlation and regression analysis to potentially indicate or conform the trends observed in the present study.
Conclusions
In conclusion, lentivirus vector-mediated overexpression of RhoGDI2 in mice ASMCs was associated with lower expressions of β2AR and GAP, while higher expressions of GEF and GRK2, parallel to reduce the β2AR density, potentially contributing to β2AR desensitization. Thus RhoGDI2 may be an important mediator of β2AR desensitization in asthmatic patients. Extensive further research, however, will be required to elucidate the full role of RhoGDI2 and other intermediates involved in β2AR desensitization prior to the development of future in vivo targets and clinical treatments.
Acknowledgements
This study was supported by Natural Science Foundation of China (NO: 30971306), Six big talent peak in Jiangsu province project (the seventh batch NO: 033), Nantong social development project (NO: S2009023) and Nantong fourth period “226 high-level personnel training project” project.
Disclosure: The authors declare no conflict of interest.
References
- Litonjua AA. The significance of beta2-adrenergic receptor polymorphisms in asthma. Curr Opin Pulm Med 2006;12:12-7. [PubMed]
- To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012;12:204. [PubMed]
- Jockers R, Angers S, Da Silva A, et al. Beta(2)-adrenergic receptor down-regulation. Evidence for a pathway that does not require endocytosis. J Biol Chem 1999;274:28900-8. [PubMed]
- Paris J, Peterson EL, Wells K, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol 2008;101:482-7. [PubMed]
- Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet 2009;374:1754-64. [PubMed]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science 2005;308:512-7. [PubMed]
- Seibold A, Williams B, Huang ZF, et al. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol Pharmacol 2000;58:1162-73. [PubMed]
- Vaughan DJ, Millman EE, Godines V, et al. Role of the G protein-coupled receptor kinase site serine cluster in beta2-adrenergic receptor internalization, desensitization, and beta-arrestin translocation. J Biol Chem 2006;281:7684-92. [PubMed]
- Ren XR, Reiter E, Ahn S, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A 2005;102:1448-53. [PubMed]
- Shenoy SK, McDonald PH, Kohout TA, et al. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001;294:1307-13. [PubMed]
- Liu H, Zhou LF, Zhang Q, et al. Increased RhoGDI2 and peroxiredoxin 5 levels in asthmatic murine model of beta2-adrenoceptor desensitization: a proteomics approach. Chin Med J (Engl) 2008;121:355-62. [PubMed]
- Zhou T, Liang B, Su GY, et al. Identification of ubiquitin target proteins using cell-based arrays. J Proteome Res 2007;6:4397-406. [PubMed]
- Holmes KD, Babwah AV, Dale LB, et al. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem 2006;96:934-49. [PubMed]
- Moore RH, Millman EE, Alpizar-Foster E, et al. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J Cell Sci 2004;117:3107-17. [PubMed]
- Plouffe BD, Radisic M, Murthy SK. Microfluidic depletion of endothelial cells, smooth muscle cells, and fibroblasts from heterogeneous suspensions. Lab Chip 2008;8:462-72. [PubMed]
- Pang L, Knox AJ. PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am J Physiol 1997;273:L1132-40. [PubMed]
- Johnson PR, Black JL, Carlin S, et al. The production of extracellular matrix proteins by human passively sensitized airway smooth-muscle cells in culture: the effect of beclomethasone. Am J Respir Crit Care Med 2000;162:2145-51. [PubMed]
- Mak JC, Nishikawa M, Shirasaki H, et al. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J Clin Invest 1995;96:99-106. [PubMed]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 2005;15:356-63. [PubMed]
- Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J 2005;390:1-9. [PubMed]
- Makita N, Kabasawa Y, Otani Y, et al. Attenuated desensitization of β-adrenergic receptor by water-soluble N-nitrosamines that induce S-nitrosylation without NO release. Circ Res 2013;112:327-34. [PubMed]
- Zhu W, Petrashevskaya N, Ren S, et al. Gi-biased β2AR signaling links GRK2 upregulation to heart failure. Circ Res 2012;110:265-74. [PubMed]
- Tesmer JJ. Structure and function of regulator of G protein signaling homology domains. Prog Mol Biol Transl Sci 2009;86:75-113. [PubMed]
- Moissoglu K, McRoberts KS, Meier JA, et al. Rho GDP dissociation inhibitor 2 suppresses metastasis via unconventional regulation of RhoGTPases. Cancer Res 2009;69:2838-44. [PubMed]
- Zhang Y, Liu H, Ni S. The research of Rho protein and beta-2 adrenergic receptor desensitization. Journal of Nantong University (Medical Sciences) 2011;1:020.
- Walker JK, Peppel K, Lefkowitz RJ, et al. Altered airway and cardiac responses in mice lacking G protein-coupled receptor kinase 3. Am J Physiol 1999;276:R1214-21. [PubMed]


