Optimal managements of stage IIIA (N2) non-small cell lung cancer patients: a population-based survival analysis
Introduction
Lung cancer has become the worldwide leading cause of cancer-related mortality nowadays and non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers (1). Among patients with NSCLC, 15% are diagnosed with stage IIIA (2-4). With the acknowledgement that mediastinal lymph node metastases (N2 staging) are associated closely with treatment and prognosis, the optimal management of stage IIIA-N2 NSCLC patients is unclear. Variable results of surgery versus radiotherapy after induction chemotherapy were reported (5,6). Nevertheless, despite surgical resection is radical and may lead to a survival benefit, whether the surgery is superior to radiotherapy is still controversial (7).
Furthermore, the difficulty of accurate clinical staging challenges the treatment scenario of those patients in this stage. According to the 7th edition AJCC TNM staging, NSCLC patients with ipsilateral mediastinal and/or subcarinal lymph node (N2) diagnosed as IIIA stage include T1–T3 (T3: tumor >7 cm or invades) and M0 (without distant metastasis) (8,9). Moreover, the precise designation of mediastinal or subcarinal lymph node also plays the critical role in the classification (10). The false positive rate of the noninvasive staging reaches 25% to 40%, and it is recently reported that the clinical nodal overstaying rate is 43%. However, the invasive method such as mediastinoscopy or biopsy is done with varying frequency and effectiveness. (11-13). Therefore, the clinical stage evaluation of IIIA-N2 NSCLC patients is needed to be more precise and the issue whether IIIA (cN2) cases are resectable or unresectable (treated mainly with chemotherapy or radiation) is still controversial.
The Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute provides information on cancer occurrences in 18 areas of United States and covers approximately 26% of the population (14). We perform this database study to evaluate the outcomes of patients treated with surgery, radiation, or both of them with different sequences. In addition, we investigate several relevant potential factors to clarify how they affect the outcome and hope to provide more information for clinical treatment decision-making.
Methods
Data collection
We conducted this retrospective study using the data retrieved from SEER database of National Cancer Institute through the SEER*Stat software version 8.3.2. For we had no access to the identities of patients included, the informed consent was not deemed as necessary in this population-based research.
Histologically confirmed NSCLC patients diagnosed between 2004 and 2011 were selected by us from SEER. Patients were further specified as being clinical stage IIIA [7th editions of the American Joint Commission on Cancer staging manual (8)] T1-3, N2, and M0. Patients with missing data for treatment were excluded. The study was approved by ethic community of Shandong Provincial Hospital afflicted to Shandong University (ID: 2017-222). All the experiments described here were performed in accordance with the approved guidelines.
We collected relevant information of those patients, which included patient ID, age, sex, characteristics of tumor (size, location, histologic type and differentiation grade) and treatment details (surgical type and radiation sequence). The outcome endpoints studied in this research are overall survival (OS) and lung cancer-specific survival (LCSS). Thus, we retrieved the vital status, cause of death and survival months of the patients.
Subgroup definitions
In this study, included patients were grouped into different sexes, ages, differentiation grades, tumor sizes and tumor locations. According to International Classification of Diseases for Oncology 3rd Edition (ICD-O-3) codes, histologic subgroups were defined as adenocarcinoma, squamous cell carcinoma and other types such as large cell carcinoma. Surgical types were classified into partial/wedge resection, lobectomy/bilobectomy and complete pneumonectomy. Moreover, based on different treatment modalities, patients were divided into five subgroups including: (I) patients with no surgery or radiation; (II) patients with surgery alone; (III) patients with radiation alone; (IV) patients with radiation prior to surgery; (V) patients with radiation after surgery.
Statistical analysis
The Kaplan-Meier method was used to estimate OS and LCSS. OS was the interval from the time of diagnosis to death of any cause, and LCSS was defined as the time from diagnosis to death of lung cancer. We analyzed the OS and LCSS as a function of 5 different treatment interventions in overall patients and each of 19 subgroups and used the log-rank test to evaluate the difference of survival between 5 treatment subgroups. Cox Proportional Hazards Regression Model was used to estimate the effects of multiple variables on survival. All tests were two-sided and P values less than 0.05 were considered to be statistically significant. All statistical analyses were conducted using SPSS statistics (version 22, IBM).
Results
Study population
Based on the inclusion criteria, 98,700 IIIA-cN2 NSCLC patients diagnosed from 2004 to 2011 were identified from SEER database, including 55,208 males and 43,492 females. Among the patients, 47,568 (48.19%) were without surgery or radiation, 5,419 (5.49%) were given surgery alone, 35,283 (35.75%) were treated with radiotherapy alone, 1,390 (1.41%) were given radiation prior to surgery, 9,040 (9.16%) were given radiation after surgery. The patient demographics and tumor characteristics were listed in Table 1.
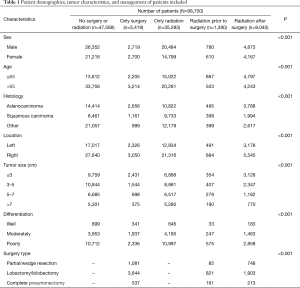
Full table
Univariate and multivariate analysis
In the Cox hazard analysis, after testing the proportional hazards assumption, we selected variables including sex, age, tumor histology, size, location, differentiation degree, surgery type and therapy. According to the result of univariate Cox-regression analysis, male, age >65, squamous and other histologic types, larger tumor, poor differentiation degree were associated with shorter OS and LCSS. Compared with the male (reference), the hazard ratio, 95% confidence interval [HR (95% CI)] of the female was 0.864 (0.853, 0.876) for OS and 0.847 (0.834, 0.860) for LCSS. In comparison with the younger (age ≤65), HR (95% CI) was 1.341 (1.323, 1.360) for OS and 1.344 (1.324, 1.365) for LCSS in elder (age >65) patients. When adenocarcinoma was considered as reference, the HR (95% CI) was 1.065 (1.047, 1.084) for OS and 1.072 (1.051, 1.094) for LCSS in squamous carcinoma patients, and other histologic types had HR (95% CI) of 1.373 (1.352, 1,393) for OS and 1.373 (1.350, 1,396). For surgical types, lobectomy/bilobectomy with HR (95% CI) of 0.576 (0.543, 0.610) for OS and 0.538 (0.503, 0.574) for LCSS and complete pneumonectomy with 0.747 (0.682, 0.817) for OS and 0.712 (0.640, 0.792) for LCSS have the better outcomes, compared with partial/wedge resection. All of P values were less than 0.05 except the location of tumor (P=0.406 for OS and 0.282 for LCSS).
In the multivariable analysis of OS and LCSS, the variables of sex, age, tumor size, differentiation degree, surgery type and therapy were statistically significant (P<0.001), with only squamous cell carcinoma (P=0.902 for OS and 0.329 for LCSS) excluded. Results of univariate and multivariate Cox regression of prognostic factors for OS and LCSS in stage IIIA (cN2) NSCLC patients were stated in Table 2.
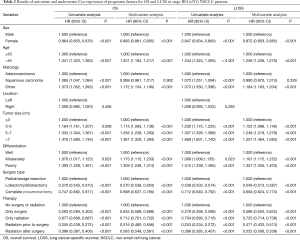
Full table
According to multivariable analysis, the outcomes of four different managements were significantly improved from that of the patients without any surgery or radiation (reference). HRs (95% CI, P) for patients given surgery alone were 0.632 (0.598, 0.668), P<0.001 for OS and 0.586 (0.550, 0.625), P<0.001 for LCSS. For patients treated with radiation alone, HRs (95% CI, P) were 0.712 (0.701, 0.722), P<0.001 for OS and 0.725 (0.714, 0.738), P<0.001 for LCSS. Among the patients given both, the HRs (95% CI, P) were 0.516 (0.480, 0.556), P<0.001 for OS and 0.471 (0.433, 0.513), P<0.001 for LCSS in patients given radiation prior to surgery and were 0.565 (0.549, 0.581), P<0.001 for OS and 0.522 (0.506, 0.539), P<0.001 for LCSS in patients treated by radiation after surgery.
Survival analysis
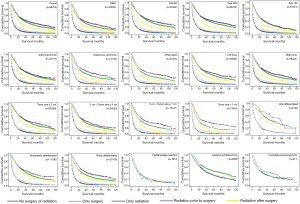
The OS, LCSS were estimated by Kaplan-Meier method (Figures 1,2), which showed that for those IIIA-cN2 patients, all of the four treatments improved the survival of patients (P<0.001). Survival of patients treated with surgery was better than that of patients treated by radiotherapy alone (P<0.001). Besides, radiation prior to surgery was the optimal sequence among the four managements. The survival analysis by log-rank test showed that there were significant differences between each two of them with every P value less than 0.001.
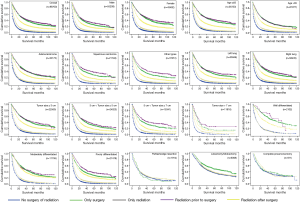
In the subgroup analysis, the same optimal order of the managements was observed in every subset. Based on the results of OS analysis, in the subgroups of age >65 (P=0.902), adenocarcinoma (P=0.279), tumor size ≤3 cm (P=0.170), well differentiated (P=0.360) patients, the survival of patients treated by radiation prior to surgery showed insignificantly difference from that of patients given surgery alone. Besides, there was no significant difference between natural outcome and outcome of patients given radiation alone (P=0.132) in well differentiation group.
According to the LCSS analysis, preoperative radiation showed significant superior to surgery alone in male (P<0.001), right lung location (P=0.019), tumor of 5–7 cm (P=0.005) and poorly differentiated (P<0.001) patients. Moreover, in well differentiated patients, compared with natural outcome, the survival improvement of radiation was insignificant (P=0.490). The P values of the overall and every subset survival analysis were listed in Table 3 for OS and Table 4 for LCSS.
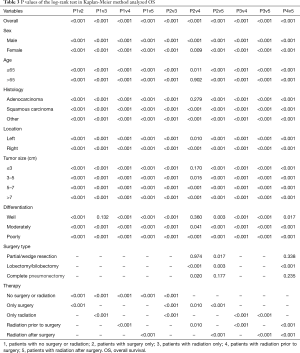
Full table
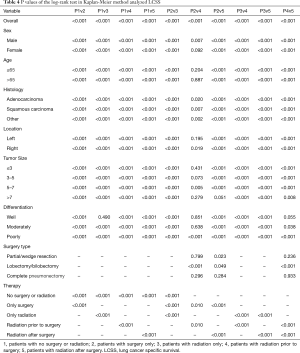
Full table
Discussion
The role of surgery in stage IIIA (N2) NSCLC patients was still controversial. Radiotherapy or surgery had been operated singly or in combination to control the local tumor. It was reported in many studies that induction treatment followed by surgery had a survival benefit in selected IIIA (N2) NSCLC patients (15-19); however, several trials had failed to demonstrate the superiority of surgery over chemoradiotherapy (20-22). The main causes of the debate were the heterogeneity of stage IIIA definition ranging from T1N2-T3N2 (8,9) and the variation of determination of N2, including pathologically proven N2 (pN2) in the resected specimen or biopsy and radiologically determined clinical N2 (23). In previous studies comparing the different treatment sequences of this stage patients, eleven trials and meta-analysis reported that postoperative radiotherapy (PORT) was detrimental to patients with cancer resection and shall not be used in the routine treatment of those patients (24-26). However, there were some other trials pointing out that PORT was no benefit or detriment to survival for stage III disease and the effect was unclear, especially in patients with N2 (27,28). Besides, one population-based study using data retrieved from National Cancer Database (NCDB) showed that the PORT improved survival of stage IIIA (pN2) NSCLC patients treated with complete resection and multi-agent chemotherapy (29).
The NCI SEER database covers approximately 28% of the US population, which will include a larger population size than other clinical trials (30). In addition, the robustness of SEER database to determine the predictors of survival outcomes has been underscored by the revisions to the NSCLC AJCC TNM classification project (9,31,32). In this effort, we analyzed the survival data of stage IIIA (cN2) patients from SEER to compare the outcomes of different treatments and explore the potential clinical factors related with prognosis.
In our study, we investigated the survival data of stage IIIA (cN2) NSCLC patients with different managements, mainly to investigate if the surgery benefited. The results showed significant differences between OS in patients without any treatments, patients with surgery only, patients with radiation only, patients with radiation before surgery and patients with radiation after surgery. The OS of patients with radiation before surgery was longer than any other managements and all four treatments were superior to natural outcomes in the overall population. In the subgroup analysis, we found that the difference was not significant between surgery alone group and radiation before surgery group in age >65, adenocarcinoma, tumor size ≤3 cm and well differentiated groups. Thus according to our results, we suggested that for patients in those subgroups, preoperative radiation was not necessary in improving the OS.
The finding about LCSS was similar to that of OS in the overall patients. The LCSS was longest in patients with radiation before surgery. All four treatments functioned for outcomes of treated patients were significantly improved compared with the untreated patients. However, the results of subgroup analysis indicated that in comparison with patients given surgery alone, preoperative radiation showed significant superiority only in male, right lung location, tumor size between 5 and 7 cm and poorly differentiated patients. In other subgroups, the effect of preoperative radiation was unobvious. Besides, in well differentiated group, radiation could not improve the prognosis, for the outcome difference between patients given radiation and untreated patients was not significant.
In the Cox-regression analysis, we concluded that male, age >65, squamous and other histologic types, larger tumor, poor differentiated patients were faced with higher death risk. Besides, the parameter of tumor location was excluded to be an independent prognostic factor. Other parameters such as age, sex and tumor size were identified meaningful factors.
However, our retrospective research was limited by the lack of some related data from the database and did not allow for randomization. The limitation of our study was mainly due to the lack of chemotherapy data of SEER. Many phase III RTOG clinical trials demonstrated chemotherapy along with radiotherapy prolonged the survival of stage III (N2) and the effect of chemotherapy could not be neglected (33). Moreover, it was possible that patients who received chemotherapy would be more likely to perform the operation, which would also cause the bias to the results favoring the surgery. Second, the radiotherapy arm of patients might be inoperable for comorbidities which also influenced their survival (34). Third, SEER database did not include many potential parameters influencing outcomes such as specific mutations, forced expiratory volume in 1 second and specific radiation technology. Besides, the infiltrative N2 status, such as the station and number of lymph nodes, was also considered to be valuable in the multi-modality treatment decision on this stage N2 patients as proposed by American College of Chest Physicians recently, which might provide more references for clinical research and practice.
In conclusion, our study showed that in SEER database, preoperative radiation with surgery was used less frequently in patients of clinical stage IIIA-cN2 disease, but had the most encouraging survival outcomes compared with radiation or surgery alone. However, in several subsets, such as the elder, adenocarcinoma, smaller tumor size and well differentiated patients, the preoperative radiotherapy was not necessary with the purpose of improving survival. In addition, the PORT was less recommended for those patients in this stage because of no significant outcome improvement. Further study was warranted to investigate the related clinical factors more comprehensively and analyze the survival benefit with the chemotherapy or tri-modality data to reach a more accurate conclusion for clinical therapy. Besides, the new treatment such as targeting therapy and immunotherapy could be evaluated and offer more options for the treatment of this stage patients in the future.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (81672288), Provincial Science and Technology Development Plan of Shandong (2015GSF118063).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethic Community of Shandong Provincial Hospital Afflicted to Shandong University. The number/ID of the ethic approval was 2017-222. All the experiments described here were performed in accordance with the approved guidelines.
References
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Mountain CF. Staging classification of lung cancer. A critical evaluation. Clin Chest Med 2002;23:103-21. [Crossref] [PubMed]
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Vansteenkiste J, Betticher D, Eberhardt W, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Thorac Oncol 2007;2:684-5. [Crossref] [PubMed]
- Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:243s-65s.
- van Meerbeeck JP. The controversial role of surgery in stage III NSCLC. Lancet Oncol 2008;9:607-8. [Crossref] [PubMed]
- Vallieres E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Hancock J, Rosen J, Moreno A, et al. Management of clinical stage IIIA primary lung cancers in the National Cancer Database. Ann Thorac Surg 2014;98:424-32; discussion 32. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 6. [Crossref] [PubMed]
- Boffa D, Fernandez FG, Kim S, et al. Surgically Managed Clinical Stage IIIA-Clinical N2 Lung Cancer in The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:395-403. [Crossref] [PubMed]
- National Cancer Institute: Surveillance, Epidemiology, and End Results. Available online: https://seer.cancer.gov
- Taylor NA, Liao ZX, Cox JD, et al. Equivalent outcome of patients with clinical Stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys 2004;58:204-12. [Crossref] [PubMed]
- Caglar HB, Baldini EH, Othus M, et al. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer 2009;115:4156-66. [Crossref] [PubMed]
- Toyooka S, Kiura K, Takemoto M, et al. Long-term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non-small-cell lung cancer with mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012;14:565-9. [Crossref] [PubMed]
- Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol 2013;8:915-22. [Crossref] [PubMed]
- Yamaguchi M, Toyokawa G, Ohba T, et al. Preoperative concurrent chemoradiotherapy of S-1/cisplatin for stage III non-small cell lung cancer. Ann Thorac Surg 2013;96:1783-9. [Crossref] [PubMed]
- Stephens RJ, Girling DJ, Hopwood P, et al. A randomised controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer 2005;49:395-400. [Crossref] [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Shien K, Toyooka S. Role of surgery in N2 NSCLC: pros. Jpn J Clin Oncol 2016;46:1168-73. [PubMed]
- Okawara G, Ung YC, Markman BR, et al. Postoperative radiotherapy in stage II or IIIA completely resected non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer 2004;44:1-11. [Crossref] [PubMed]
- Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 1998;352:257-63. [Crossref] [PubMed]
- Feng QF, Wang M, Wang LJ, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys 2000;47:925-9. [Crossref] [PubMed]
- Burdett S, Rydzewska L, Tierney J, et al. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2016;10:Cd002142. [PubMed]
- Lee HW, Noh OK, Oh YT, et al. Radiation Therapy-First Strategy After Surgery With or Without Adjuvant Chemotherapy in Stage IIIA-N2 Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;94:621-7. [Crossref] [PubMed]
- Herskovic A, Mauer E, Christos P, et al. Role of Postoperative Radiotherapy in Pathologic Stage IIIA (N2) Non-Small Cell Lung Cancer in a Prospective Nationwide Oncology Outcomes Database. J Thorac Oncol 2017;12:302-13. [Crossref] [PubMed]
- Kelly RJ, Force J, Rajan A, et al. Evaluation of Kras mutations and angiogenic biomarkers in patients with advanced non-small cell lung cancer (NSCLC) receiving single-agent sorafenib (S). J Clin Oncol 2010;28:7626. [Crossref]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [Crossref] [PubMed]
- Ball D, Mitchell A, Giroux D, et al. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 2013;8:315-21. [Crossref] [PubMed]
- Johnstone DW, Byhardt RW, Ettinger D, et al. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:365-9. [Crossref] [PubMed]
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic significance of tumor size in patients with stage III non-small-cell lung cancer: a surveillance, epidemiology, and end results (SEER) survey from 1998 to 2003. J Thorac Oncol 2012;7:1479-84. [Crossref] [PubMed]