
Endoscopic vein harvesting: technique, outcomes, concerns & controversies
Introduction
Despite increasing recognition of benefits of multiple arterial grafting (1-3), long saphenous vein (LSV) remains a frequently chosen conduit for coronary artery bypass grafting (CABG) (4). Traditionally, LSV is harvested using a lengthy incision in the lower limb termed open vein harvesting (OVH). More recently, endoscopic vein harvesting (EVH) has grown in popularity in an effort to reduce the pain and risk of infection associated with the procedure (5). The Society of Thoracic Surgeons’ National Cardiac Database reported that 70% of CABG procedures performed in 2008 used this vein harvesting method (6). Abundant evidence has emerged in recent years confirming that EVH is associated with decreased leg wound morbidity, improved cosmetic results, and enhanced patient satisfaction (4,7-9). Despite these well-established benefits, concerns persist regarding risk of injury at the time of EVH with its potential detrimental effect on vein graft patency and clinical outcomes (10). This review article comprehensively deals with the technical aspects, outcomes, concerns, and controversies associated with EVH.
Technique of EVH
Several disposable and reusable EVH systems with and without carbon dioxide insufflation are available, including the most frequently used disposable systems: VasoView HEMOPROTM (Maquet Holding GmbH & Co.), VirtuoSaphTM (Terumo Cardiovascular Systems Corporation., USA), and ClearGlide® (Sorin, USA). After proper positioning of the patient, the location of the vein is identified by the operator through gentle “milking” of the vein, while feeling the thrill with the other hand. If the operator is unable to palpate the vein, osseous landmarks or a portable, point of care ultrasound machine (SonoSite Inc., USA) can be used for localization. Through a three cm incision just above the medial aspect of the knee, 35 cm of the thigh leg LSV may be harvested. If the entire 70 cm length of vein is needed, the options are to repeat the procedure through the same incision in the other direction, or to begin the harvesting procedure 2-3 cm above the medial malleolus followed by a second incision just above the knee to harvest the vein to the groin. After the vein is identified, a balloon tip trocar is inserted into the incision and the tunnel is inflated with carbon dioxide. The conical dissection cone is advanced toward the groin on the anterior surface of the vein under videoscopic visualization. Circumferential blunt dissection of the vein is completed along the posterior and lateral aspects throughout its length, after which the collateral branches are isolated and divided with bipolar electrocautery. Vein trauma is minimized by constant visualization, proper counter-traction, and careful hemostasis. Proximal saphenous vein ligation is performed through a separate “stab and grab” incision at the extremity of the tunnel. Once the vein is extracted, the proximal end is cannulated and gently dilated to avoid endothelial trauma. The branches are doubly clipped and avulsions are repaired with fine monofilament suture material. The vein is gently flushed to remove any clots that may have accumulated. Finally, the tunnel is evacuated of residual blood, a redivac drain is placed and after wound closure the leg is wrapped with a compression bandage for at least 48 hours (Video 1).
Technical issues
The presence of retained clot within the LSV lumen has been an increasingly recognized complication of EVH (11). Brown and colleagues used optical coherence tomography (OCT) for intraoperative assessment of the entire tract of the harvested LSV to investigate the effect of preheparinization and sealed carbon dioxide insufflation on intraluminal clot burden (12). Preheparinization before EVH (either a 5,000-IU bolus or full-dose heparin to achieve an activated clotting time >300 seconds) resulted in significant reductions in clot fraction and clot volume. Additionally, significant reductions in clot burden were observed after EVH with an open CO2 insufflation system without preheparinization. An open CO2 system may be achieved by using either the ClearGlide® system or, as is performed at author’s institution, the VasoViewTM device without balloon inflation, such that CO2 insufflation is used to facilitate visualization, but without a sealed tunnel. These findings support the hypothesis that clot formation during EVH is the result of stagnant blood that is not anticoagulated being allowed to remain within a collapsed vein. There was no difference in this study among the three intervention groups: (I) low-dose and (II) full-dose preheparinization and (III) an open CO2 insufflation system without preheparinization. Although this study was not powered to determine whether intraluminal clot strands predispose to graft failure, the author’s view is that every effort should be made to standardize and optimize vein quality during EVH and that preheparinization and/or an open CO2 insuflation system should be used to reduce clot burden. In a similar study, Burris et al. demonstrated significantly higher endothelial integrity and lower tissue factor activity in veins that were not distended with saline compared to those that were distended using syringe injection with no method of controlling the distending pressure (11). Again, avoidance of over distension cannot be overemphasized.
Outcomes
A large amount of evidence has emerged in recent years in the form of randomized controlled trials (9,13-21) (Table 1), observational studies (5,8,22,23), as well as systematic reviews and meta-analyses (7,24-30) demonstrating that EVH can be safely used as an alternative to open techniques in CABG. The available evidence (5-9,13-21) predominantly confirms that EVH is no worse than OVH at short- and mid-term follow-up.
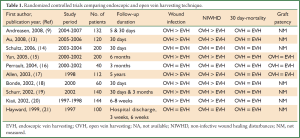
Full table
Wound infection
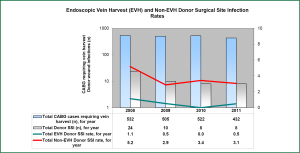
Infective wound complications associated with long incisions used to harvest the LSV are common and well documented. The incidence of infective wound complications following traditional OVH ranges from 2-25%, resulting in significant economic and clinical burden. A meta-analysis of eleven randomized controlled trials reported a significant reduction in wound infection using an endoscopic technique (OR 0.22, 95% CI; 0.14-0.37, P<0.00001), for a number needed to treat of fourteen (30). Sastry et al. (24) in a more recent meta-analysis of 31 studies [15 prospectively randomized (PR), 7 prospective non-randomized (PNR) and 9 retrospective non-randomized (RNR)] also indicated less wound infection in the EVH groups (SRRisk 0.31, 95% CI; 0.23-0.42, P<0.0001), and this was confirmed after excluding non-randomized data (SRRisk 0.26, 95% CI; 0.15-0.44, P<0.0001). At the author’s institution EVH has been associated with a significant reduction in rate of donor site infection compared to OVH. A recently published 4 years follow-up of EVH from the author’s institution confirms that adoption of EVH has almost eliminated donor site infection compared to OVH (0.39% vs. 3.9%, P<0.001) as shown in Figure 1 (5). The reduction in donor site infections likely occurs because of preserved tissue perfusion and a lower likelihood of creating vital tissue flaps than with open saphenectomy. This may be of particular importance in patients with diabetes, obesity, and peripheral vascular disease in whom EVH has eliminated the excessive risk observed with traditional OVH (17,28).
Non-infective wound healing disturbances (NIWHD)
NIWHD include wound drainage, hematoma, dehiscence, necrosis, need for surgical debridement, and seroma formation. The morbidity that results from these not only leads to increasing re-intervention rates and treatment costs, but also ultimately results in impaired mobilisation, increased pain, and patient dissatisfaction. This can in turn potentially result in an increased length of hospital stay. Athanasiou et al. (31) in a meta-analysis of 27 studies (both randomised and non-randomised) showed that the NIWHD of hematoma, edema/swelling, skin necrosis, dehiscence/separation, wound drainage, and seroma/lymphocele are all reduced with EVH when compared to OVH. Similar outcomes have been reported by a more recent and much bigger meta-analysis (24). It is important to emphasize that these non-infective wound complications may predispose to infection of the leg wound following harvest of the LSV. An example of this is wound dehiscence or separation, which can impair tissue healing by affecting tissue apposition, thereby predisposing to wound infection. Similarly wound hematoma may act as an infective focus following surgery. The reduction in wound-related morbidity following EVH may have several elements, including reduced trauma to surrounding tissues, less inflammation, fewer disturbances to skin vascularization, and finally avoidance of skin flap creation.
Postoperative pain, mobility, and patient satisfaction
Several studies have demonstrated improved quality of life indices, including postoperative pain, time to mobilization, and patient satisfaction following EVH. The incidence of pain (23.1% versus 6.7%), neuralgia (24.3% versus 7.1%), and patient satisfaction (49% versus 75%) was significantly improved with EVH compared with OVH (29). Using a visual analogue scale, patients undergoing EVH rated their experience of pain two points lower on a 0-10 scale throughout the entire postoperative period and labeled themselves pain-free days earlier than their OVH counterparts (18,20,32). A number of studies have reported earlier mobility for patients undergoing EVH as well as improved mobility at hospital discharge and six weeks after surgery (20,33). Patient satisfaction with the cosmetic result is initially higher with EVH than after traditional harvest, although the cosmetic outcome is equivalent six weeks after surgery (20).
Hospital length of stay (LOS) and costs
Earlier mobilization of patients and a reduction of wound complications may result in a reduction of recovery time and therefore hospital LOS. The results in small series have not been consistent, although two meta-analyses have reported a reduction in hospital LOS by EVH with a weighted mean difference ranging from –0.85 to –1.04 days; 95% CI: –1.92 to –0.16 (29,31). In an economic analysis of cost-effectiveness, Rao and colleagues calculated health related quality of life (HRQol) utility estimates and reported that EVH was more cost-effective than OVH (34). The authors estimated the HRQoL utility on discharge to be 0.9443 after EVH and 0.6815 after OVH. Six weeks postoperatively, the utility was 0.9599 after EVH and 0.8219 after OVH. By using these calculated utility estimates, it was demonstrated that EVH is a cost-effective alternative to conventional OVH. The ICER of $19,858.87/QALY compared favorably with other health care interventions. Probabilistic sensitivity analysis demonstrated with a 95.6% certainty that EVH was the most cost-effective technique at a cost-effectiveness threshold of $50,000/QALY. Alternative analysis demonstrated that even with a high degree of uncertainty associated with the true value of the incremental QALY payoff (±50%), EVH was more cost-effective than conventional OVH, with a certainty of 67.6%.
Postoperative myocardial infarction (within 30 days of CABG)
Sastry et al. (24) recently undertook a meta-analysis of twelve studies (3 PR, 4 PNR and 5 RNR) that reported on postoperative myocardial infarction (MI) in a total of 1872 patients. Analysis of all 12 studies did not indicate a difference in postoperative MI between the two groups (SR Risk 0.87, 95% CI: 0.68-1.11, P=0.26). The results were similar after excluding the non-randomized data (SR Risk 1.34, 95% CI: 0.30-5.89, P=0.70). No significant difference was found between the EVH and OVH groups in postoperative MI incidence. The type of device used for EVH was found to have no significant influence on the overall summary effect size: Ethicon compared with VasoView (P=0.71); other devices compared with VasoView (P=0.77); other devices compared with Ethicon (P=0.83).
Vein graft stenosis
Three studies (2 PR and 1 RNR) included in a recently published meta-analysis (24) considered vein graft stenosis, reporting on a total of 3,229 patients. In the two prospective studies, angiography was performed at three, and six months. In the RNR study, angiograms were reviewed at a median 12.6 months after CABG. The SR ratio was 1.19, 95% CI: 1.05-1.34, P=0.005. However, neither of the randomized studies showed any significant difference between the groups. In a recently published 4 years follow-up study from the author’s institution (5) at a mean duration of follow-up of 26.4±10.3 months, vein graft occlusion rate was 11.3%, with an additional 6.3% demonstrating stenotic regions of greater than 50% for those patients who underwent voluntary graft patency assessment. Inclusion of 30 patients who underwent symptom-guided graft patency assessment of their 60 vein grafts, with subsequent repeat revascularization, increased the vein graft occlusion rate to 11.5% with 9.5% grafts demonstrating stenotic regions of greater than 50%. More importantly, no significant patency differences were noted between EVH and OVH veins. In this study the overall 6-month vein graft occlusion rate was 14.1%, with an additional 11.4% demonstrating stenotic regions of greater than 50%. No significant patency differences were noted between EVH and OVH veins. These figures are similar to those reported by Perrault and colleagues (16) and better than those reported by Yun and associates (15).
In conclusion, the evidence for increased rates of vein graft stenosis after EVH is very weak. More studies are needed to investigate this further.
Vein graft occlusion
The meta-analysis of four studies (2 PR, 1 PNR and 1 RNR) reported on vein graft occlusion in a total of 4,700 patients (24). Analysing the data from all four studies, the SR ratio was 1.39 (95% CI: 1.11-1.75, P=0.004), suggesting a higher rate of vein graft occlusion in the EVH group. However, when considering only the two randomized control trials the difference in vein graft occlusion between the groups was non-significant. It was not possible to assess the influence of devices on overall effect size due to low numbers of studies (only two studies had non-missing information on devices), and the fact that both remaining studies used the VasoView system. In conclusion, the evidence for increased rates of vein graft occlusion after EVH is very weak.
Angina recurrence
Sastry et al. in their recently published meta-analysis assessed angina recurrence reported in four studies (2 PR, 1 PNR and 1 RNR) including a total of 6,401 patients (24). In the 2 PR studies, the follow-up was 6 months. In the PNR study, the median follow-up was 2.6 years. In the remaining study, median follow-up was 17 months after EVH and 37 months after OVH. The rate of angina recurrence was not significantly different between groups (SR ratio 1.06, 95% CI: 0.49-2.25, P=0.81), even after removing the non-randomized studies (SR ratio of 0.79, 95% CI: 0.15-4.18, P=0.78). However, it is important to emphasize that at present there is insufficient evidence to show that the rate of angina recurrence is any different between the EVH and OVH groups.
Repeat revascularization
Sastry et al. (24) in their meta-analysis compared the incidence of repeat revascularization reported in seven studies (1 PR, 2 PNR and 4 RNR), including a total of 21,743 patients. One of the RNR studies did not publish the duration of follow-up. In the other seven studies, the median follow-up was 2.3 years. The SR ratio was 1.16, 95% CI: 0.99-1.36, P=0.06, indicating insufficient evidence of any difference in the repeat revascularization rate. The one randomized study yielded an SR ratio of 0.34, 95% CI: 0.01-8.25 after continuity correction, indicating no significant difference between groups. These conclusions were unchanged after performing a sensitivity analysis excluding studies reporting hazard ratios (SR ratio 1.19; 95% CI: 0.96-1.46; P=0.12).
30-day mortality
Sastry et al. in their recently published meta-analysis of sixteen studies (7 PR, 4 PNR and 5 RNR) compared the impact of EVH versus OVH on 30-day mortality in a total of 14,190 patients. The SRRisk was 0.71, 95% CI: 0.56-0.90, P=0.005, indicating a lower incidence of 30-day mortality in the EVH group. Exclusion of the non-randomized data resulted in SRRisk 0.75, 95% CI: 0.27-2.11, P=0.58. In summary, although there was a trend towards 30-day survival benefit in EVH over OVH groups, there was no clear evidence for this when considering randomized controlled trials only. The choice of device was found to have no significant influence on the overall summary effect size: Ethicon compared with VasoView (P=0.50); other devices compared with VasoView (P=0.75); other devices compared with Ethicon (P=0.83).
Mid-term mortality
Currently, there is insufficient evidence to demonstrate a difference in mid-term mortality between the EVH and OVH groups. A recent meta-analysis of ten studies (1 PR, 2 PNR and 7 RNR) compared mid-term mortality reporting on a total of 252 915 patients over a median follow-up of 22.5 months. The SR ratio was 0.90, 95% CI: 0.79-1.03, P=0.12, which indicates insufficient evidence for a difference in mid-term mortality. There was substantial heterogeneity between the studies (I2-statistic 47%). The findings remained unchanged after sensitivity analysis excluding studies with hazard ratios (SR ratio 0.90; 95% CI: 0.68-1.19; P=0.45). Also, the only randomized study reported a rate ratio of 3.00 with 95% CI: 0.13-71.52; P=0.50.
Concerns & controversies
Vein graft failure
Lopes et al. (10) were the first to challenge the use of EVH as a routine surgical approach and called into question whether its use may expose patients to the risk of vein graft failure, death, myocardial infarction, and repeat revascularization. In a secondary analysis of 3,000 patients from the PREVENT IV trial, Lopes and colleagues found an association of EVH with higher rates of vein graft failure (46.7% vs. 38.0%, P<0.001) and adverse clinical outcomes including mortality (HR 1.52; P=0.005) (10). This study was designed to assess ex-vivo treatment of vein grafts with edifoligide, and patients were not randomly assigned to harvest procedure. In the absence of randomization, outcomes cannot be definitively attributed to harvest technique. Furthermore, the study was conducted at 107 sites between 2002 and 2003, and a number of technical factors were not standardized, including technician experience, institutional EVH volume, and details of the harvest technique.
Zenati and colleagues also published a secondary analysis of a randomized trial, the Randomized On/Off Bypass (ROOBY) trial, designed to evaluate differences in clinical outcomes between patients undergoing on- and off-pump CABG (35). Of the 2203 patients recruited into the original trial, 1,471 (66.8%) had conduit data recorded and 894 (40.6%) had angiographic follow-up at one year. These latter two groups formed the basis of the subgroup analysis, in which the authors found inferior rates of vein graft patency and increased repeat revascularization rates in the EVH group. This study has limitations similar to the subgroup analysis of the PREVENT IV trial. Both studies were recruiting patients in the early part of the last decade, when EVH uptake in the US was low (<10%). The variability in experience levels, the effect of the learning curve, and the potentially low number of cases per institution or practitioner should be considered when interpreting these findings. Furthermore, data regarding technical details about conduit harvest and intraoperative flow characteristics were unfortunately not recorded during these studies. These variables are well-recognized to affect graft patency. Finally, it is important to emphasize that the primary purpose of these studies was not to compare vein harvest techniques. Surgeons were encouraged to use whichever harvesting technique they preferred, and a selection bias may exist with unmeasured confounders affecting surgeons’ decision to use an EVH approach.
Learning curve
A significant institutional and personal learning curve exists upon adoption of the EVH technique. Macroscopic lesions, such as holes or torn side branches requiring suture repairs, occur 3-5 times more often after EVH than after open harvest, and concern exists regarding the overall reduced ability of abiding by “no-touch” principles and preserving the vasa vasorum when handling vascular tissue endoscopically (36). Desai and colleagues compared veins harvested by an open technique to those harvested by EVH performed by experienced versus novice technicians using optical coherence tomography (OCT) to image veins intraoperatively and computed tomographic angiography (CTA) to assess graft patency on post-operative day 5 (37). They found that technicians inexperienced with EVH (<100 cases of experience) were far more likely to provoke deep vessel injury when compared with those with more than 900 cases of experience. Furthermore, when the number of discrete injures exceeds a threshold of 4, the risk of early graft failure rises by more than 50% (67% versus 96% graft patency at five days; P=0.05). In this well-designed study, intimal injury diagnosed by OCT correlated with endothelial disruption on histological examination and the expression of tissue factor activity on the luminal surface, supporting the hypothesis that focal injury with disruption of the endothelium can provoke an occlusive thrombus and loss of the graft.
Kiani et al. (38) in a further sophisticated study investigated the impact of learning curve on the quality and early function of endoscopically harvested LSV. EVH was performed during CABG by “experienced” (>900 cases, n=55 patients) vs. “novice” (<100 cases, n=30 patients) technicians. Afterwards, conduits were and examined for vascular injury using OCT, with segments identified as injured further examined for gene expression using a tissue injury array. Conduit diameter was measured intra- and postoperatively (day 5 and 6 months) using OCT and CTA. It was evident that EVH performed by novice harvesters resulted in increased number of discrete graft injuries and higher expression of tissue injury genes. Regression analysis revealed an association between shear stress and early dilation (positive remodeling) (R2=0.48, P<0.01). Injured veins showed blunted positive remodeling at 5 days and a greater degree of late lumen loss at 6 months.
These studies (37,38) confirm that vein graft quality is dependent on technician experience, and careful attention must be paid to assuring safe and reliable training of technicians at the beginning of their learning curve. Most of the recent CABG studies that reported adverse outcomes after EVH were enrolling patients while this technique was relatively new and being rapidly adopted (16,17,35,39). Although not reported in these studies, it is likely that a minority of the harvesters had more than 100 cases of experience during this timeframe. Differences in graft patency and clinical outcomes compared with the OVH technique may regress towards the mean over time if inexperience is the only cause of problems. However, many institutions are continually training new physician assistants to perform this technique. This means that inexperience with EVH can continue to impact outcomes even at institutions that have extensive familiarity with the technique.
Currently, there is no consensus on the standard number of cases required for technicians to traverse the learning curve. However, there is a general agreement that simulation training before the implementation of EVH in real world practice is likely to improve outcomes compared with “learning by doing” (40).
Conclusions
EVH is being rapidly adopted as a routine surgical approach at many cardiac surgical centers worldwide. This rapid adoption of EVH is prompted by a dramatic reduction in invasiveness compared with traditional OVH technique. Available evidence confirms two- and three-fold improvements in the rate of wound-related complications and infections for EVH. The significant reduction in incision length when grafts are procured using EVH yields less wound-related pain thereby translating into increased patient satisfaction. Although the required disposable equipment costs are increased, shortened length of hospital stay, elimination of leg wound infections and a reduction in NIWHD compared with OVH maintains the cost-effectiveness of EVH.
On the other hand, the association between this technique and poor graft patency has recently been called into question. Reassuringly, available evidence does not suggest that EVH is an inherently poor method for procuring vascular conduits. However, poor conduit quality, a consequence of the learning curve for EVH, has been shown to be a predictor of early graft failure, blunted positive remodeling, and greater negative remodeling. Given the ongoing annual volume of CABG procedures that utilize EVH, the learning curve for this procedure represents an important and under-recognized public health issue. There is a stronger need for adopting strategies aimed at minimising the negative impact of learning curve on vein graft quality. It is expected that further evidence in future in the form of long-term follow-up studies will prove a major contributor in addressing the misperceptions and misconceptions associated with EVH and enhance its universal adoption.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Raja SG, Siddiqui H, Ilsley CD, et al. In-hospital outcomes of off-pump multivessel total arterial and conventional coronary artery bypass grafting: single surgeon, single center experience. Ann Thorac Surg 2009;88:47-52. [PubMed]
- Locker C, Schaff HV, Dearani JA, et al. Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery: analysis of 8622 patients with multivessel disease. Circulation 2012;126:1023-30. [PubMed]
- Dimitrova KR, Hoffman DM, Geller CM, et al. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg 2012;94:475-81. [PubMed]
- Raja SG, Haider Z, Ahmad M, et al. Saphenous vein grafts: to use or not to use? Heart Lung Circ 2004;13:403-9. [PubMed]
- Raja SG, Rochon M, Sproson C, et al. 4-year outcome analysis of endoscopic vein harvesting for coronary artery bypass grafting. ISRN Vascular Medicine Volume 2013, Article ID 517806, 8 pages. Available online: http://dx.doi.org/
10.1155/2013/517806 - Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg 2009;88:S2-22. [PubMed]
- Deppe AC, Liakopoulos OJ, Choi YH, et al. Endoscopic vein harvesting for coronary artery bypass grafting: a systematic review with meta-analysis of 27,789 patients. J Surg Res 2013;180:114-24. [PubMed]
- Ouzounian M, Hassan A, Buth KJ, et al. Impact of endoscopic versus open saphenous vein harvest techniques on outcomes after coronary artery bypass grafting. Ann Thorac Surg 2010;89:403-8. [PubMed]
- Andreasen JJ, Nekrasas V, Dethlefsen C. Endoscopic vs open saphenous vein harvest for coronary artery bypass grafting: a prospective randomized trial. Eur J Cardiothorac Surg 2008;34:384-9. [PubMed]
- Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med 2009;361:235-44. [PubMed]
- Burris N, Schwartz K, Brown J, et al. Incidence of residual clot strands in saphenous vein grafts after endoscopic harvest. Innovations (Phila) 2006;1:323-7. [PubMed]
- Brown EN, Kon ZN, Tran R, et al. Strategies to reduce intraluminal clot formation in endoscopically harvested saphenous veins. J Thorac Cardiovasc Surg 2007;134:1259-65. [PubMed]
- Au WK, Chiu SW, Sun MP, et al. Improved leg wound healing with endoscopic saphenous vein harvest in coronary artery bypass graft surgery: a prospective randomized study in Asian population. J Card Surg 2008;23:633-7. [PubMed]
- Schultz SC, Stapleton D, D’Ambra P, et al. Prospective randomized study comparing the Teleflex Medical SaphLITE Retractor to the Ethicon CardioVations Clearglide Endoscopic System. J Cardiothorac Surg 2006;1:24. [PubMed]
- Yun KL, Wu Y, Aharonian V, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: six-month patency rates. J Thorac Cardiovasc Surg 2005;129:496-503. [PubMed]
- Perrault LP, Jeanmart H, Bilodeau L, et al. Early quantitative coronary angiography of saphenous vein grafts for coronary artery bypass grafting harvested by means of open versus endoscopic saphenectomy: a prospective randomized trial. J Thorac Cardiovasc Surg 2004;127:1402-7. [PubMed]
- Allen KB, Heimansohn DA, Robison RJ, et al. Influence of endoscopic versus traditional saphenectomy on event-free survival: five-year follow-up of a prospective randomized trial. Heart Surg Forum 2003;6:E143-5. [PubMed]
- Bonde P, Graham A, MacGowan S. Endoscopic vein harvest: early results of a prospective trial with open vein harvest. Heart Surg Forum 2002;5:S378-91. [PubMed]
- Schurr UP, Lachat ML, Reuthebuch O, et al. Endoscopic saphenous vein harvesting for CABG -- a randomized, prospective trial. Thorac Cardiovasc Surg 2002;50:160-3. [PubMed]
- Kiaii B, Moon BC, Massel D, et al. A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery. J Thorac Cardiovasc Surg 2002;123:204-12. [PubMed]
- Hayward TZ 3rd, Hey LA, Newman LL, et al. Endoscopic versus open saphenous vein harvest: the effect on postoperative outcomes. Ann Thorac Surg 1999;68:2107-10; discussion 2110-1. [PubMed]
- Kirmani BH, Barnard JB, Mourad F, et al. Mid-term outcomes for Endoscopic versus Open Vein Harvest: a case control study. J Cardiothorac Surg 2010;5:44. [PubMed]
- Felisky CD, Paull DL, Hill ME, et al. Endoscopic greater saphenous vein harvesting reduces the morbidity of coronary artery bypass surgery. Am J Surg 2002;183:576-9. [PubMed]
- Sastry P, Rivinius R, Harvey R, et al. The influence of endoscopic vein harvesting on outcomes after coronary bypass grafting: a meta-analysis of 267 525 patients. Eur J Cardiothorac Surg 2013; [Epub ahead of print]. [PubMed]
- García-Altés A, Peiró S. A systematic review of cost-effectiveness evidence of endoscopic saphenous vein harvesting: is it efficient? Eur J Vasc Endovasc Surg 2011;41:831-6. [PubMed]
- Markar SR, Kutty R, Edmonds L, et al. A meta-analysis of minimally invasive versus traditional open vein harvest technique for coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2010;10:266-70. [PubMed]
- Cadwallader RA, Walsh SR, Cooper DG, et al. Great saphenous vein harvesting: a systematic review and meta-analysis of open versus endoscopic techniques. Vasc Endovascular Surg 2009;43:561-6. [PubMed]
- Reed JF 3rd. Leg wound infections following greater saphenous vein harvesting: minimally invasive vein harvesting versus conventional vein harvesting. Int J Low Extrem Wounds 2008;7:210-9. [PubMed]
- Cheng D, Allen K, Cohn W, et al. Endoscopic vascular harvest in coronary artery bypass grafting surgery: a meta-analysis of randomized trials and controlled trials. Innovations (Phila) 2005;1:61-74. [PubMed]
- Athanasiou T, Aziz O, Skapinakis P, et al. Leg wound infection after coronary artery bypass grafting: a meta-analysis comparing minimally invasive versus conventional vein harvesting. Ann Thorac Surg 2003;76:2141-6. [PubMed]
- Athanasiou T, Aziz O, Al-Ruzzeh S, et al. Are wound healing disturbances and length of hospital stay reduced with minimally invasive vein harvest? A meta-analysis. Eur J Cardiothorac Surg 2004;26:1015-26. [PubMed]
- Coppoolse R, Rees W, Krech R, et al. Routine minimal invasive vein harvesting reduces postoperative morbidity in cardiac bypass procedures. Clinical report of 1400 patients. Eur J Cardiothorac Surg 1999;16:S61-6. [PubMed]
- Patel AN, Hebeler RF, Hamman BL, et al. Prospective analysis of endoscopic vein harvesting. Am J Surg 2001;182:716-9. [PubMed]
- Rao C, Aziz O, Deeba S, et al. Is minimally invasive harvesting of the great saphenous vein for coronary artery bypass surgery a cost-effective technique? J Thorac Cardiovasc Surg 2008;135:809-15. [PubMed]
- Zenati MA, Shroyer AL, Collins JF, et al. Impact of endoscopic versus open saphenous vein harvest technique on late coronary artery bypass grafting patient outcomes in the ROOBY (Randomized On/Off Bypass) Trial. J Thorac Cardiovasc Surg 2011;141:338-44. [PubMed]
- Ouzounian M, MacPherson C, Ali IS. Endoscopic versus open vein harvesting for coronary artery bypass grafting. In: Raja SG, Amrani M. eds. Off-pump coronary artery bypass grafting: outcomes, concerns & controversies. Nova: New York, 2012: 331-42.
- Desai P, Kiani S, Thiruvanthan N, et al. Impact of the learning curve for endoscopic vein harvest on conduit quality and early graft patency. Ann Thorac Surg 2011;91:1385-91; discussion 1391-2. [PubMed]
- Kiani S, Desai PH, Thirumvalavan N, et al. Endoscopic venous harvesting by inexperienced operators compromises venous graft remodeling. Ann Thorac Surg 2012;93:11-7; discussion 17-8. [PubMed]
- Dacey LJ, Braxton JH Jr, Kramer RS, et al. Long-term outcomes of endoscopic vein harvesting after coronary artery bypass grafting. Circulation 2011;123:147-53. [PubMed]
- Kiani S, Poston RS. Reply. Ann Thorac Surg 2013;95:383. [PubMed]


