Post-traumatic acute thoracic aortic injury (TAI)—a single center experience
Introduction
The damage of the heart and large vessels is diagnosed in even 30% of victims of serious injure and it is second after head trauma cause of multiorgan trauma-related deaths (1). Thoracic aortic injury (TAI) occurs in 2% of cases with blunt thoracic trauma and often is considered as a life-threatening injury (2). Damage to these structures occurs mostly as a result of traffic accidents and a degree of aortic wall injury is directly proportional to the energy (3). The vast majority (even up to 90%) of patients with aortic injury die at the scene of accident or during transport to the hospital. However, at least of 70% of those who reach the emergency departments can be saved.
The consequences of major aortic injuries can be critical. The sudden hemorrhage, hypovolemic shock, and consequently death affect 85% of victims (3,4). In the remainders self-limiting hematoma or pseudoaneurysm may develop (5). The mortality rate of victims with TAI who reach the hospitals can be decreased to approximately 5% if appropriate surgical or percutaneous techniques are applied. There is no doubt that TAI among accident survivors represents a direct threat to life and requires fast-track diagnostic and therapeutic pathways (3-6).
The aim of our study was to assess the safety and outcomes of endovascular treatment with stentgrafts of TAI in multiorgan trauma patients. The primary outcomes were technical successful deployment of stent graft and in-hospital mortality. The secondary outcomes included procedural local complications, neurological adverse events such as stroke or spinal cord ischemia (SCI) and left arm claudication.
Methods
Patients
Since 2007 to July 2017 we have performed endovascular treatment [(thoracic endovascular aortic repair (TEVAR)] of the thoracic aorta pathologies in Department of Cardiac Surgery and Transplantology in Poznan in 114 patients. In 15 (14 men and 1 woman) of them (13% of all patients treated with intravascular techniques) with the median age of 30 years (18–64 years), emergency implantation of a stent graft for TAI was necessary.
According to the rules of Local Bioethical Committee of our university the Statement of Ethics Approval is not required for retrospective data analysis of patients treated with the use of standard methods.
Preoperative examinations
Patients after accidents were firstly admitted to the emergency departments of the regional hospitals. Then after examinations including trauma scan and echocardiography were performed and as soon as TAI was recognized, patients were transferred immediately to our department (the only high reference center for endovascular treatment of thoracic aortic aneurysms and injuries in 6 million inhabitants macroregion).
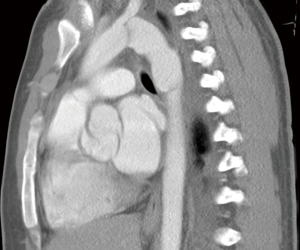
Every patient was diagnosed by means of computed tomographic (CT) scan with contrast medium injection (CT angiography) (Figure 1). The analysis of CT angiographic scans of TAI included: type of injury, aortic arch anatomy, vertebral artery dominance, existence of atherosclerosis, length of aortic wall injury, aortic diameter at the site of injury as well as of proximal and distal segments to aortic pathology, distance between the left subclavian artery (LSA) origin and planned landing zone (LZ) of stent graft.
Stent graft implantation procedure
Each endovascular procedure was performed as an emergency operation in the endovascular operating C-arm unit (Allura, Philips Medical Systems, Best, The Netherlands). Available image intensifier field sizes were 17, 23, and 31 cm. Generally, procedures were performed with the use of ionic contrast medium (non-ionic one was reserved for patients with allergy and pre-existing renal failure). The injection of contrast medium was performed with the use of an automatic syringe. Five to ten series were performed, depending on clinical indications. Before surgery, the patients received 5,000 IU of heparin, except one case with a fracture of the skull base. In all individuals, antibiotic prophylaxis was used according to the approved hospital protocol. No spinal cord protection methods were employed.
The medical team consisted of two cardiac surgeons, intervention radiologist and anesthesiologist.
Postoperative evaluation
After the emergency endovascular procedures patients were routinely transferred to our postoperative intensive care unit (ICU) to monitor vital organs and systems performance. If there were no obvious local complications and basic life functions were stable, patients were discharge from our hospital and referred to the specialized trauma centers.
In the first year after surgery, patients were regularly followed up in outpatient facilities in first month after procedure and every three months. During clinical follow-up examinations at 1, 3, 6 and 12 months after stent graft implantation controlled computed tomography angiography (CTA) was also performed.
Data management
Because of no normal distribution the results are presented as medians with the range (minimum–maximum).
Results
The cause of TAI was traffic accident in 13 cases and in 2 was falling from a height, including the collapse of an ultralight aircraft (Table 1). The Injury Severity Scale (ISS) in 80% (12 cases) was greater than 40. Eight patients admitted to our department were conscious [Glasgow Coma Scale (GCS) >12)], the 7 was unconscious, under sedation, intubated and mechanically ventilated.

Full table
The indications for implantation of a stent graft were rupture of the thoracic aortic wall (grade IV) in 9 cases (60%) and aortic pseudoaneurysm (grade III) in 6 cases (40%).
Due to the serious state of the victims in 13 cases we decided on general anesthesia. In two subjects, only a local anesthesia was applied because of perforation of the trachea in one patient and of spinal injury in cervical segment in another one.
Thoracic stent graft implantations were performed with surgical access, through the femoral arteries. The left common femoral artery was punctured by Seldinger’s, and the right one was exposed surgically. We implanted in 12 cases a Zenith (Cook Medical, Bloomington, Inc., USA), in 3 cases GORE TAG (Gore Medical, Flagstaff, USA) stent grafts. In eleven cases, the proximal end of the stent graft was positioned in the 3 and 4 LZs, in four cases in LZ 2 covering the orifice of the LSA.
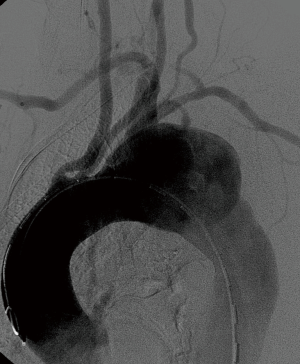
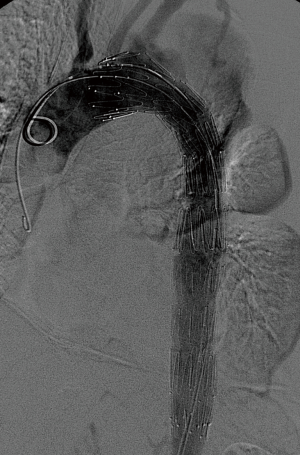
There were no complications during the procedure, and the oversizing of the stent graft relative to the aorta not exceeded 10%. The control angiography demonstrated laminar flow of blood through the prosthesis and the thoracic aorta and complete exclusion of the injured aortic segment (Figures 1-3). The median of graft length was 140 mm (134–160 mm) and a diameter 28 mm (24–34 mm) (Table 2).
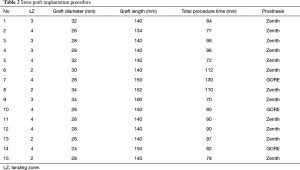
Full table
All patients survived the intervention without any additional procedures or conversion to open surgery. Immediately after the treatment no patient had symptoms of SCI or other complications associated with the implantation of the stent graft. The total time of the procedure was 87 minutes (70–130 minutes) and the total fluoroscopy time did not exceed 25 minutes and the maximum dose 1 Gy.
All patients survived the hospital stay in Cardiac Surgery Department and were referred to traumatic departments for treatment of additional stable multiorgan injuries. In the early period after implantation of the stent graft the Velazquez syndrome (fever and leukocytosis but without markers of infection such as high sensitivity C-reactive protein and procalcitonin) was observed in five cases.
The early mortality within 30 days was 0/15 (0%). In almost each case the patient’s length of stay after surgery in the Department of Cardiac Surgery does not exceed 24 hours. Overall survival for the cohort was 100% and in follow-up all the patients were still alive. None of them required secondary intervention because of TAI-related complications and the technical success rate was 100%.
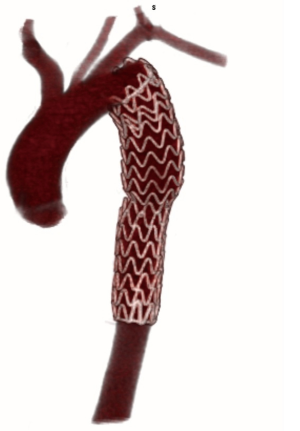
The follow-up period was 6–108 months and control examinations included CTA at 1, 3, 6, 12 months after procedure, and later on once a year (Figure 4). It has been shown in each case the correct position and prosthesis function, complete exclusion of aortic wall pathology without leak evidences. There were neither migration or break the graft. Moreover, no cases of aorta dilatation and the formation of false aneurysms were noted in the treated area.
Discussion
The medical history and physical examination may play an important role in diagnosis of aortic lesions. Crucial is interview of emergency medical team for the mechanism and energy trauma. International and Advanced Trauma Life Support (ITLS, ATLS) programs indicates the use of a history and exam during both primary and secondary surveys. The possible signs are pulse deficit in lower extremities or left arm, unexplained hypotension, hypertension in upper part compared with lower one. There are also indirect symptoms that may indicate the extent of the injury such as fracture of the first rib, shoulders, sternum and pleural effusion. Up to 50% of victims presents no external signs of chest trauma and the most important factor is diagnosis based on mechanism of injury (7).
The CT trauma scan and focused assessment with sonography for trauma (FAST) ultrasound are the investigations of choice in multiorgan trauma patients at the emergency departments. However, the highest sensitivity in the diagnosis of aortic injury has CTA. CTA has a sensitivity of 98% and specificity nearly 100% for the TAI diagnosis. The definitive signs of TAI are: active contrast extravasation, rupture, intramural hematoma, dissection, pseudocoarctation (abnormality of aortic contour). The suggestive signs are: mediastinal or periaortic hematoma, retrocrural hematoma, critical aorta caliber reduction (6,8-11). After TAI confirmation, a systolic blood pressure should be kept below 100 mmHg (8-10).
The CT angiography is also critical for the planning of TAI treatment and should include: type of injury, aortic arch anatomy, vertebral artery dominance, existence of atherosclerotic disease, length of aortic injury, aortic diameter, LSA origin location and LZ of 20 mm in length (11). The diameter of optimal stent graft should exceed the diameter of the aorta by approximately 10% (prosthesis oversizing). According to Tokyo Consensus, determination of the intended location of the stent graft positioning LZ includes a minimum 20 mm distal and proximal aortic pathology-free segments (12,13).
The extent of damage to aortic wall is a result of the deceleration or crushing forces during blunt trauma due to the difference in pliancy between the ascending, arch and descending aorta, and aortic isthmus fixed around the ligament tension (5). Depending on the location of damage TAI can be identified at the ascending part, aortic arch and the descending aorta. In the first two cases, the result of torsion forces is the formation of “water-hammer effect” —that inhibit the flow of fluid in the vessel with increased intravascular pressure back flow (6). These cases are rare, and include 7% of aortic injuries. Injury of the descending aorta usually occurs as a consequence of thoracic aorta crush between the spine and the chest wall or as a result of hyperextension of the thoracic spine, which constitutes 93% of aortic injuries (5).
Up to now several classifications of TAI have been proposed. The most popular and widely used grading system is Azizzadeh classification: grade I: intimal tear; grade II: intramural hematoma or intimal flap; grade III: pseudoaneurysm; grade IV: free rupture (14). It was also endorsed by Society of Vascular Surgery (SVS). Other classifications are the Vancouver (15); Presley Trauma Center (16) or Harborview one (17).
The minor aortic lesions term describes injuries that are minor in nature—Azizzadeh grade I: intimal tears. This injury was estimated to be present in 10–30% cases of TAI but unfortunately there is no universal definition of this lesion. Most studies used 1 cm criteria as the definition of small injury (17,18). In our center we never observe patients with minor aorta lesions. For minimal aortic injures (MAI) the SVS guidelines suggested the noninvasively individualized therapy including heart rate, blood pressure control, proper anticoagulant and antiplatelet agents. The SVS also suggests the serial CT angiographic follow-up at 1,3,6 and 12 months after the injury (19).
Endovascular techniques for treatment of TAI were used first successfully in 1997 by Semba (20). The conventional surgical repair exposes the patient to marked drop in blood pressure and significantly longer procedures. These factors contribute to more frequent postoperative cardiopulmonary failure and neurological adverse events such as paraplegia. The overall mortality rate following surgical intervention of the descending aorta is much higher than the risk of endovascular procedures (20-24).
Previous studies showed that increasing implementation of endovascular stents use did lead to decrease in early complications incidence and reduced hospital stay (20-25). The treatment of endovascular stent graft implantation is a relatively novel therapeutic method to treat TAI. According to our knowledge, there are currently no long-term studies with follow up period 10–15 years that could assess function of stent graft (25-28).
Surgical treatment of lesions of the ascending aorta include classical surgery from median sternotomy with the use of extracorporeal circulation and at least mild hypothermia while repair of descending aorta requires wide thoracotomy (14). The conventional surgical methods of reconstruction of the descending part is still accompanied by very high operative risk (15,16). Alternative endovascular treatments (TEVAR), may enable to avoid surgical risk, particularly among patients with critical, risk factors and comorbidities (20-29). The highest volume TEVAR comparison with open surgery in TAI cases was reported by Demetriades and colleagues. The TEVAR group patients received less blood transfusion and manifested lower mortality rate and shorter in-hospital stay (29).
The only treatment of Grade III–IV before the era of endovascular procedures were extensive classical surgical procedures with a high risk of mortality ranging between 15% and 45% (22,30-32). Additionally, open repair with graft interposition carried a few percent risk of paraplegia (31,32). Moreover, all of traumatic patients usually present other severe injuries and the application of extracorporeal circulation with high-dose heparin administration may result in severe adverse events and postpone treatment of the associated injuries. Alternative endovascular technique was shown to have mortality rate of 7,5% and minimal neurological complications (paraplegia close to 0% and stroke around 1.5%) in TAI subjects (33).
The decision for urgent or delayed intervention repair should be based on combination of patient specific risk of aortic rupture, grade of injury and imaging characteristics. In stable aortic injuries—grade II and III the timing is usually predicted by the severity of associated non-aortic injuries. The patients without serious additional trauma with stable aortic injuries should be performed within 24 hours of admission to avoid potential progression to grade IV—rupture. The minimal invasive endovascular TEVAR allow for earlier intervention in stable patients and emergency treatment of unstable patients (29,34).
We should be aware that even endovascular repair of TAI is associated with a risk of serious complications. The most important are endoleaks, stent graft break or displacement and retrograde aortic dissection (31). A clinical problem in the treatment of pathologies of the descending aorta is high risk of ischemic spinal cord injury (SCI, spinal cord ischemia), which is 21% in the case of open surgery, and from 2% to 12% among patients treated endovascular (35,36). The total length of the prosthesis covering the descending aorta longer than 205 mm was proved to increase the risk of spinal ischemia with consecutive paraplegia and additionally elevates the pressure of cerebrospinal fluid (37). In our group, no such long stent grafts were implanted thus we luckily avoided SCI.
In case of necessity to position the graft in LZ2 the coverage of the LSA is a relatively safe maneuver (38-40). The revascularization procedures of the LSA are only necessary in about 10% of the treated cases. There are known absolute indications such as diminutive, hypoplastic or absent right subclavian artery (RSA), left internal mammary artery coronary bypass graft, patient left axillary—femoral bypass graft, functioning left arm arteriovenous shunt for hemodialysis, aberrant origin and rare variants of the anatomical origin of the left vertebral artery (LVA) (40-42).
In the group treated in our Department, we have not observed steal syndrome or left upper limb ischemia requiring revascularization. All patients stayed strictly monitored several hours after the procedures. Clinical check-up at ICU included also examination of the left upper extremity perfusion with sonography.
The meta-analysis of Hajibandeh et al. proved that the routine LSA revascularization was not found to significantly reduce neurologic or mortality in patients undergoing TEVAR with coverage of LSA (43). The Belczak et al. reported very low incidence of arm ischemia-related adverse events after LSA coverage without subsequent revascularization. The randomized trials are required to prove the routine revascularization strategy (44).
In the early period after implantation of the stent graft the Velazquez syndrome was observed in less than 40% that was consistent with previously published reports (9,10,24,25).
A limitation of endovascular treatment of diseases of the aorta is also its diameter that should be between 22 to 46 mm to match commercially available stent grafts. One should note that the maximal oversizing of the graft should not exceed 10–15% (Tokyo Consensus) the size of the aorta. Oversizing is necessary to provide adequate radial force to keep deployed stent graft in place and prevent from endoleak development around the graft (45,46). Exceeding these values significantly increase the risk of aortic rupture or dissection. On the other side, difficulties may occur also in patients with aortic diameter of less than 18 mm, where the open surgery seems to be the only solution. We realize that the group of 15 patients is not large. However, it is a very rare treated disease and group number is comparable to the ones cited in this article.
Conclusions
In our department, techniques of TEVAR with stent-graft implantation have become methods of choice in treatment of traumatic TAIs since they have enabled to minimize operation-related risk of mortality and morbidity, particularly in unstable multitrauma patients in severe clinical status. TEVAR for TAI performed in emergency settings provide favorable long-term results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: According to the rules of Local Bioethical Committee of our university the Statement of Ethics Approval is not required for retrospective data analysis of patients treated with the use of standard methods
References
- Jabłonka S. Obrażenia serca i dużych naczyń klatki piersiowej. In: Kołodzieja J. Urazy klatki piersiowej. pod red. Warszawa 2004:101-19.
- Ungar TC, Wolf SJ, Haukoos JS, et al. Derivation of a clinical decision rule to exclude thoracic aortic imaging in patients with blunt chest trauma after motor vehicle collisions. J Trauma 2006;61:1150-5. [Crossref] [PubMed]
- Richens D, Field M, Neale M, et al. The mechanism of injury in blunt traumatic rupture of the aorta. Eur J Cardiothorac Surg 2002;21:288-93. [Crossref] [PubMed]
- Fattori R, Russo V, Lovato L, et al. Optimal Management of Traumatic Aortic Injury. Eur J Vasc Endovasc Surg 2009;37:8-14. [Crossref] [PubMed]
- Smith RS, Chang FC. Traumatic rupture of the aorta: Still a lethal injury. Am J Surg 1986;152:660-3. [Crossref] [PubMed]
- Nagpal P, Mullan BF, Sen I, et al. Advances in imaging and management trends of traumatic aortic injuries. Cardiovasc Intervent Radiol 2017;40:643-54. [Crossref] [PubMed]
- American College of Surgeons. Advanced Trauma Life Support Student Manual-9th Ed. Chicago: American College of Surgeons 2012.
- Brady WJ, Aufderheide TP, Kaplan PA. Obrazowanie układu sercowo-naczyniowego. In: Schwartz D.T., Reisdorff E.J., Radiologia wypadkowa, Lublin 2002:567-603.
- Juszkat R, Jemielity M, Pukacki F, et al. Własne doświadczenia w leczeniu śródnaczyniowym różnych schorzeń aorty piersiowej. Pol Przeg Chir 2006;78:552-65.
- Ramaiah V, Rodriguez-Lopez J, Diethrich EB. Endografting of the thoracic aorta. J Card Surg 2003;18:444-54. [Crossref] [PubMed]
- Steenburg SD, Ravenel JG, Ikonomidis JS, et al. Acute traumatic aortic injury: imaging evaluation and management. Radiology 2008;248:748-62. [Crossref] [PubMed]
- Mitchell RS, Ishimaru S, Ehrlich MP, et al. First International Summit on Thoracic Aortic Endografting: roundtable on thoracic aortic dissection as an indication for endografting. J Endovasc Ther 2002;9:II98-II105. [Crossref] [PubMed]
- Criado FJ, Clark NS, Barnatan MF. Stent graft repair in the aortic arch and descending thoracic aorta: a 4-year experience. J Vasc Surg 2002;36:1121-8. [Crossref] [PubMed]
- Azizzadeh A, Keyhani K, Miller CC 3rd, et al. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg 2009;49:1403-8. [Crossref] [PubMed]
- Gavant ML. Helicant CT grading of traumati aortic injuries. Impact on clinical guidelines for medical and surgicalmanagement. Radiol Clin North Am 1999;37:553-74. [Crossref] [PubMed]
- Lamarche Y, Berger FH, Nicolaou S, et al. Vancouver simplified grading system with computed tomographic angiography for blunt aortic injury. J Thorac Cardiovasc Surg 2012;144:347-54. [Crossref] [PubMed]
- Heneghan RE, Aarabi S, Quiroga E, et al. Call for a new classification system and treatment strategy in blunt aortic injury. J Vasc Surg 2016;64:171-6. [Crossref] [PubMed]
- Forman MJ, Mirvis SE, Hollander DS. Blunt thoracic aortic injuries:CT characterization and treatment outcomes of minor injury. Eur Radiol 2013;23:2988-95. [Crossref] [PubMed]
- Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011;53:187-92. [Crossref] [PubMed]
- Semba CP, Kato N, Kee ST, et al. Acute rupture of the descending thoracic aorta: repair with use of endovascular stent grafts. J Vasc Interv Radiol 1997;8:337-42. [Crossref] [PubMed]
- Leurs LJ, Bell R, Degrieck Y, et al. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Endograft registries. J Vasc Surg 2004;40:670-9. [Crossref] [PubMed]
- Buz S, Zipfel B, Mulahasanovic S, et al. Conventional surgical repair and endovascular treatment of acute traumatic aortic rupture. Eur J Cardiothorac Surg 2008;33:143-49. [Crossref] [PubMed]
- Arthurs ZM, Starnes BW, Sohn VY, et al. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: An analysis of the National Trauma Databank. J Vasc Surg 2009;49:988-94. [Crossref] [PubMed]
- Xenos ES, Abedi NN, Davenport DL, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg 2008;48:1343-51. [Crossref] [PubMed]
- Xenos ES, Minion DJ, Davenport DL, et al. Endovascular versus open repair for descending thoracic aortic rupture: institutional experience and meta-analysis. Eur J Cardiothorac Surg 2009;35:282-6. [Crossref] [PubMed]
- Chakfe N, Dieval F, Riepe G, et al. Influence of the textile structure on the degradation of explanted aortic endoprostheses. Eur J Vasc Endovasc Surg 2004;27:33-41. [Crossref] [PubMed]
- Szmidt J, Galazka Z, Rowinski O, et al. Late aneurysm rupture after endovascular abdominal aneurysm repair. Interact Cardiovasc Thorac Surg 2007;6:490-4. [Crossref] [PubMed]
- Buczkowski P, Puślecki M, Stefaniak S, et al. Pregnancy and childbirth in a patient after multistep surgery and endovascular treatment of cardiovascular disease. Cardiovasc J Africa 2016;27:69-70. [Crossref]
- Demetriades D, Velmahos GC, Sealea TM, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries results of American Associationfor the Surgery of Trauma multicenter study. J Trauma 2008;64:561-70. [Crossref] [PubMed]
- Fabian TC, Richardson DJ, Croce MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American association for the surgery of trauma. J Trauma 1997;42:374-80; discussion 380-3. [Crossref] [PubMed]
- Von Oppell UO, Dunne TT, DeGroot MK, et al. Traumatic aortic rupture: twenty-year metaanalysis of mortality and risk of paraplegia. Ann Thorac Surg 1994;58:585-93. [Crossref] [PubMed]
- Hunt JP, Baker CC, Leatz CW, et al. Thoracic aortic injuries: management and outcome of 144 patients. J Trauma 1996;40:547-55. [Crossref] [PubMed]
- van der Zee CP, Vainas T, van Brussel FA, et al. Endovascular treatment of traumatic aortic lesions. A systematic review and meta-analysis. J Cardiovasc Surg (Torino) 2017. [Epub ahead of print]. [PubMed]
- Eusanio MD, Folesani G, Berretta P, et al. Delayed management of blunt traumatic aortic injury: open surgical versus endovascular repair. Ann Thorac Surg 2013;95:1591-7. [Crossref] [PubMed]
- Kaya A, Heijmen RH, Rousseau H, et al. Emergency treatment of the thoracic aorta: results in 113 consecutive acute patients (the Talent Thoracic Retrospective Registry). Eur J Cardiothorac Surg 2009;35:276-81. [Crossref] [PubMed]
- Setacci F, Sirignano P, De Donato G, et al. Endovascular thoracic aortic repair and risk of spinal cord ischemia: the role of previous or concomitant treatment for aortic aneurysm. J Cardiovasc Surg (Torino) 2010;51:169-76. [PubMed]
- Amabile P, Grisoli D, Giorgi R, et al. Incidence and determinants of spinal cord ischaemia in stent-graft repair of the thoracic aorta. Eur J Vasc Endovasc Surg 2008;35:455-61. [Crossref] [PubMed]
- Kotelis D, Geisbüsch P, Hinz U, et al. Short and midterm results after left subclavian artery coverage during endovascular repair of the thoracic aorta. J Vasc Surg 2009;50:1285-92. [Crossref] [PubMed]
- Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997-1003. [Crossref] [PubMed]
- Moore RD, Brandschwei F. Subclavian-to-carotid transposition and supracarotid endovascular stent graft placement for traumatic aortic disruption. Ann Vasc Surg 2001;15:563-6. [Crossref] [PubMed]
- Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. [Crossref] [PubMed]
- Riesenman PJ, Farber MA, Mendes RR, et al. Coverage of the left subclavian artery during thoracic endovascular aortic repair. J Vasc Surg 2007;45:90-4; discussion 94-5. [Crossref]
- Hajibandeh S, Hajibandeh S, Antoniou SA, et al. Meta-analysis of left subclavian artery cowerage with and without revascularization in thoracic endovascular repair. J Endovasc Ther 2016;23:634-41. [Crossref] [PubMed]
- Belczak SQ, Silva ES, Klajner R, et al. Type II endoleaks, left-arm complications, and need of revascularization after left subclavian artey coverage for thoracic aortic aneurysm endovascular repair: a systematic review. Ann Vasc Surg 2017;41:294-9. [Crossref] [PubMed]
- Mitchell RS, Ishimaru S, Criado FJ, et al. Third international summit on thoracic aortic endografting: lessons from long-term results of thoracic stent-graft repairs. J Endovasc Ther 2005;12:89-97. [Crossref] [PubMed]
- Puślecki M, Buczkowski P, Perek B, et al. Hybrid procedures for aortic arch repair. Kardiochi Torakochi Pol 2011;4:438-44.