Robotically assisted totally endoscopic coronary artery bypass surgery
Introduction
While other surgical disciplines were highly successful and efficient in adopting endoscopic approaches for several operations, cardiac surgery (and in particular coronary artery bypass surgery) had to wait for the development of sophisticated technology which allowed performance of delicate surgical maneuvers inside the chest. Early attempts with endoscopic technology in coronary bypass surgery using conventional long shafted endoscopic instruments had completely failed (1). Surgical robotic technology emerged with the goal of performing remote operations in limited spaces and cavities. The basic parts of these systems include a surgical console or “master unit” (where the surgeon sits and controls the surgical arms and instruments), a “slave unit” (the robot itself, with three or four arms, that physically get in contact with the patient carrying the robotic surgical instruments) and a video system with screens positioned in the operating room, that share the surgeons view through a 3D/HD endoscopic camera with the rest of the surgical team. The robotic camera allows for enhanced visibility through the camera port with up to ten times magnification. The robotically controlled surgical instruments are inserted through instrument ports and are connected to the robotic arms via connector plates. All essential instrumentation is available. A specific feature is that the instruments can be moved with more degrees of freedom than conventional surgical instruments. This allows difficult endoscopic surgical maneuvers such as sewing intestinal or vascular anastomoses in narrow spaces. Until now totally endoscopic coronary bypass surgery, which can be regarded as the surgically least invasive version of coronary bypass surgery, is only possible using robotic technology, specifically the daVinci, daVinci S, and daVinci Si sytems (Intuitive Surgical, Sunnyvale CA, USA).
In totally endoscopic coronary artery bypass grafting (TECAB), the avoidance of a thoracotomy or sternotomy maximally preserves the thoracic integrity and function of the patient. This is seen as the main advantage of this ultimate minimally invasive approach.
The world’s first TECAB with robotic assistance was performed in 1998 by Loulmet et al. (2) with the first-generation da Vinci robotic system (Intuitive Surgical, Mountain View, CA, USA) on the arrested heart. A left internal mammary artery (LIMA) to left anterior descending artery (LAD) anastomosis was performed successfully. Over the following years, subsequent interest and enthusiasm motivated surgeons to perform more complex operations, leading to the development of multivessel TECAB, off-pump operations, and hybrid approaches (TECAB combined with percutaneous coronary interventions).
The development of newer and better surgical telemanipulators certainly contributed to that: high definition video, improved arm movement, addition of a fourth arm, better instrument reach, faster instrument changes, development of robotic coronary stabilizers, and a dual console system for teaching purposes (3). Still the development is relatively slow and only a few centers worldwide currently perform robotic TECAB. The main reasons for this fact are: high complexity of operations and corresponding long learning curves, lack of an endoscopic surgical tradition in the cardiac surgical community as well as cost and lack of reimbursement in some countries.
This article reviews the main advances of this surgical approach over the recent years and gives an outline of the current status of TECAB.
Brief history
Many investigators started using the robot in a stepwise approach, performing only part of the surgery with robotic assistance and later on moving to a totally endoscopic operation. While in 1998 totally endoscopic LIMA to LAD grafting was already performed using the daVinci system, the preclinical experiments of Stephenson et al. (4) in isolated pig hearts allowed the development of a robotic surgical anastomosis technique without the help of an assistant utilizing the Zeus Robotic Microsurgical System (Computer Motion). Zeus used long shafted conventional endoscopic instruments mounted on robotic arms. In 1999, Ducko et al. (5) reported LIMA harvesting and coronary anastomosis between LIMA and LAD on calves under cardiopulmonary bypass (CPB), also using the Zeus system. All anastomosis were patent. In the year 2000 Damiano et al. (6) reported on the first US Food and Drug Administration (FDA) trial of feasibility and efficacy of robotic technology (Zeus Robotic Microsurgical System) to perform LIMA-LAD anatomosis during open chest conventional surgery. Out of ten patients, eight had perfect functioning anastomosis detected by Ultrasonic flow measurements and two needed to be reconstructed manually. Long term follow up of these ten patients showed 100% patency by coronary angiogram. The Zeus system, however, never had a breakthrough in totally endoscopic CABG because instrument dexterity was inadequate. Also in 2000, Kappert et al. (7) reported on the world’s first bilateral IMA (BIMA) TECAB with the first generation daVinci system. Using femoral bypass and arrested heart with the help of an endoballon for ascending aortic occlusion and antegrade cardioplegia delivery, this group was able to perform in-situ LIMA to the obtuse marginal (OM) branch and in-situ right IMA (RIMA) to the LAD. Cichon et al. (8) reported a series of 17 patients where both IMAs were harvested robotically and the anastomosis were performed through a 6-8 cm right thoracotomy on the arrested heart. The first BIMA TECAB done in the beating heart was reported by Farhat et al. in 2004 utilizing an octopus TE stabilizer which was mounted on the rails of the operating table (9).
The first report on a large series on the use of robotic technology to perform CABG operations came in 2001 from Leipzig, Germany (10). The robotic system was utilized in 131 patients (LIMA harvesting or coronary anatomosis). In 35 the procedure was attempted in a totally endoscopic fashion (27 on-pump, and eight off-pump). Of the 27 on-pump patients, five (18%) required conversion to full sternotomy. The long term patency rate of this group was 95%. On the other hand, in the beating heart group, six out of eight patients (75%) needed conversion to full sternotomy. Kappert in 2001 (11) was able to complete TECAB in beating heart in 78% of 37 patients (LIMA-LAD) with 100% survival rate.
In 2006, a very important step was taken: the FDA-sanctioned multicenter trial on the safety and efficacy of the da Vinci Surgical System (Intuitive Surgical) for TECAB was finally reported (12). Overall 98 patients requiring single vessel LAD revascularization were enrolled in 12 centers. All aspects of surgery were to be performed with the robot (LIMA take down, intracorporeal coronary anastomosis). All procedures were done under femoral-femoral CPB, arrested heart with the use of endoballon and totally endoscopic approach. Thirteen patients were excluded during surgery due to failed femoral cannulation or inadequate intrathoracic working space. In the 85 patients who had the TECAB procedure completed, CPB time was 117±44 min, cross-clamp time was 71±26 min, and hospital length of stay (LOS) was 5.1±3.4 days. Conversion to open techniques was necessary in five patients (6%). No deaths or strokes were observed, one early reintervention was necessary (1.5%) and one peri-operative myocardial infarction (MI) occurred (1.5%). Three month coronary angiograms were performed in 76 patients: significant (>50%) stenosis or total occlusions of the anastomosis were found in six patients (8%). At this time point total freedom from reintervention or angiographic failure was 91%. In conclusion this multicenter trial showed the safety and efficacy of the procedure, with no deaths, low morbidity and acceptable LIMA patency rates (13). Further larger series on single and multivessel revascularization were reported later. The first triple vessel totally endoscopically robotic operation on the arrested heart was reported in 2010 (14).
General conduct of the operation
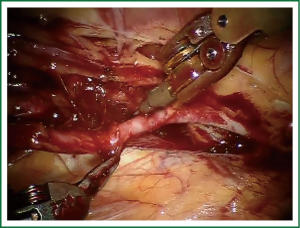
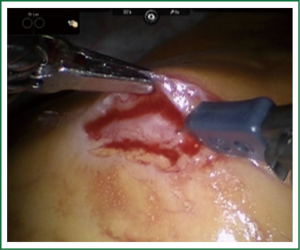
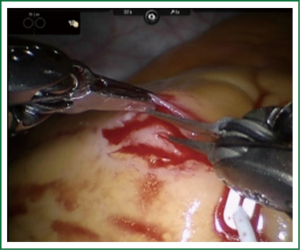
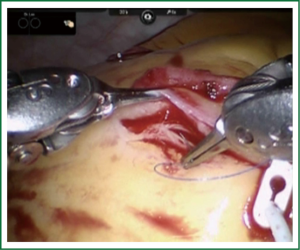
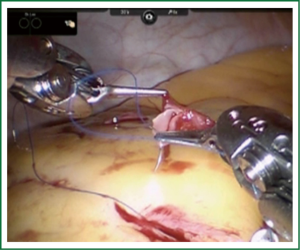
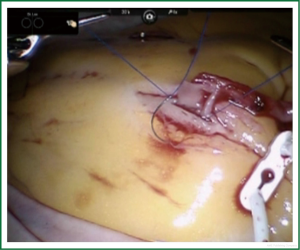
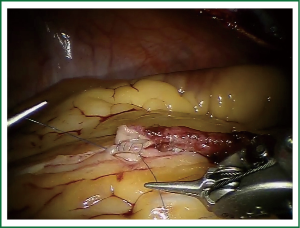
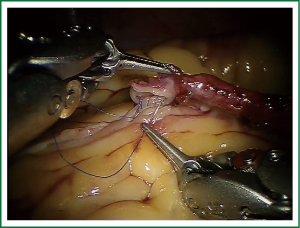
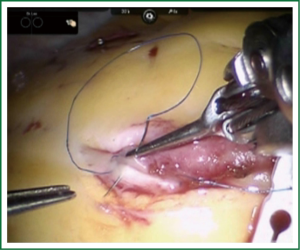
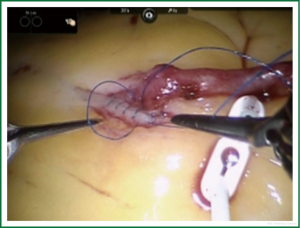
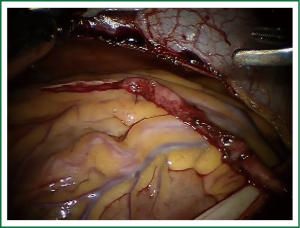
TECAB is most commonly performed with the robotic arms docked to the patient’s left chest. With the left lung collapsed a camera port is inserted into the 5th intercostal space, instrument ports are inserted into the third and seventh intercostal spaces. After docking the internal mammary arteries are harvested (Figure 1). Both vessels can be taken down with the same port arrangement. Most surgeons perform IMA harvesting in skeletonized technique. After pericardial fat pad removal and opening of the pericardium the target vessels are exposed. Graft-to-coronary anastomoses are performed using polypropylene sutures (Figures 2-11), U-clips, or anastomotic connectors. Flow measurements are carried out with endoscopic transit time flow probes. After assurance of adequate graft quality and inspection for hemostasis the robotic system is undocked and chest tubes are inserted.
TECAB on the arrested heart
Arresting the heart for performing TECAB offers the advantage of easier handling of a flaccid heart. By deflating both lungs on CPB the intra-thoracic space is improved and all three coronary systems can be reached.
CPB is installed in the groin and an endoballoon positioned in the ascending aorta is used to induce cardioplegia. Operation time and efficiency can be optimized in arrested heart TECAB (AHTECAB) if the steps of endoscopic IMA harvesting and femoral vessel dissection and cannulation are performed at the same time by two surgeons, one at the console and the other at the operating table side.
One femoral artery should be used for both CPB and introduction of the endoballoon, if the artery can accommodate a 23 Fr cannula. Alternatives are using both femoral arteries (one for CPB and the other for the endoballon with smaller cannulas in both sides) or sewing an 8 mm Hemashield graft to the left axillary artery for CPB and using one 19 Fr cannula on a femoral artery for endoballoon insertion.
The endoaortic occlusion balloon is advanced up to the ascending aorta, with transesophageal echocardiographic (TEE) guidance. CPB is started, the endo-occlusion balloon is inflated in the ascending aorta (keeping the balloon pressure up to 350 mmHg) The endoballoon has a distal channel that allows antegrade high potassium cold cardioplegia to be delivered in the aortic root. Cardiac arrest can be rapidly induced using injections of adenosine.
Safe use of the endoaortic occluder balloon requires the presence of a right radial or brachial invasive arterial blood pressure catheter. The reason is the occasional dislodgement of the balloon with possible occlusion of the innominate artery.
A recent review (15) of published series on TECAB (on-pump and off-pump) found 14 articles with good quality data and outcome report to perform a systematic review. The group of AHTECAB consisted of 360 patients from four publications (16-19). In this large group all-cause mortality was 0.4%, perioperative stroke and MI were 0.8% and 1.8% respectively, new onset atrial fibrillation 5.1%, renal failure 1.2%, early reintervention 2.3% and revision for bleeding 5.8%. From the 253 patients who had some form of imaging study of the grafts soon after surgery the patency rate was 96.4%. These results compare favorably with conventional approaches, with the exception of reoperation for bleeding which seems higher.
Intermediate and long term follow up data are scarcer in the literature. An 8 years’ follow up on 82 patients by Currie et al. (20) (with coronary angiography, CTA and stress myocardial perfusion scintigraphy) found a 92.7% overall patency rate in a mixed on-pump and off-pump population. Regarding LIMA-LAD anastomosis the patency rate was 93.4%. Bonatti et al. in 2012 (21) showed a clinical follow up of five years on 62 one-vessel disease patients reporting a survival of 95.8%, freedom from major adverse cardiac and cerebrovascular events (MACCE) of 83.1% and freedom from angina of 91.1%.
Although in the early years of robotic experience most operations performed on the arrested heart were isolated LIMA-LAD anastomosis either as treatment of single vessel disease or part of a hybrid procedure (with PCI to non-LAD targets), recently more multivessel bypasses have been performed. The first small series (ten patients) was published in 2007 (22). Patients received BIMA to the LAD and circumflex system, under arrested heart. Conversion rate was 30%, median intensive care unit (ICU) LOS was 41 h and median hospital LOS was 7 days. No cardiac or cerebrovascular events were observed. Sequential bypasses and Y-graft constructions can be safely performed in a totally endoscopic environment (19). When BIMA are being used, the usual approach is the LIMA being anastomosed to an OM branch and the RIMA, crossing the midline, being anastomosed to the LAD. Exposure of OM branches is achieved by rotating the heart to the right with the use of the Intuitive EndoWrist stabilizer (Intuitive Surgical, Sunnyvale, CA), controlled by the fourth arm of the robot which is docked to the left subcostal space. The length of both skeletonized IMAs is usually adequate to reach both targets. Triple bypass surgery on the arrested heart is feasible (14). If the PDA needs to be bypassed it is usually done so by a Y graft of radial artery from the LIMA-LAD. The inferior wall of the heart can be reached with the help of the coronary stabilizer brought in through the left instrument port.
TECAB on the beating heart
Beating heart TECAB (BHTECAB) was first performed in the early 2000s (23) and should be part of the armamentarium of any team who performs TECAB. Although off-pump CABG has lost some popularity recently due to concern on long term patency of grafts (24), it certainly offers short term benefits in a selected population. In the context of TECAB, a beating heart operation should be considered when there is any severe difficulty to attain cannulation or endoaortic balloon occlusion. Small femoral and axillary vessels can preclude the safe use of cannulas for remote access CPB. More than mild calcification of the ascending aorta should be seen as a contraindication for the use of the endoaortic occluder device, as well as an aorta larger than 4 cm. Patients with severe kidney dysfunction and vasculoppathy might benefit from the avoidance of a CPB run.
The position of ports and technique for IMA harvesting is similar to AHTECAB. After opening of the pericardium and visualization of the LAD, a left subcostal port is inserted for the use of the endostabilizer. The robotic endostabilizer comes as a robotic instrument on the daVinci S and Si systems and works as a suction stabilizer. As compared to the earlier OctopusTETM it can be fully controlled by the robotic surgeon at the console. This device improved considerably the ability to perform safe coronary anastomosis on the beating heart: it transforms a moving target into a non-moving one, offers saline squirt once the coronary is open and can also help immobilize the heart for non-LAD targets.
The same systematic review mentioned earlier (15) found 880 patients submitted to BHTECAB published in literature. Overall results include: all cause mortality of 1.2%, stroke of 0.7%, MI of 0.8%, new atrial fibrillation of 10.7%, renal failure of 4.4%, early reintervention rate of 2.6% and revision for bleeding 2.6%. In 659 grafts studied, the patency rate was 98.3%. A comparison with AHTECAB with the current data is difficult since there might be selection bias in both populations. Apparently BHTECAB presented with higher short term mortality than AHTECAB (1.2% versus 0.4%), lower reoperation for bleeding (2.6% versus 5.8%) and good short term patency rate. Srivastava et al. (25) reported their more recent experience with BHTECAB where there was no conversion in 164 single and multivessel patients. Mortality, stroke and MI were 0.6% each and graft patency was 99.5% in the short term. Of note all anastomosis were created either with U clips or automatic devices. A recent publication from the University of Chicago including data from the same group, however, showed reasonable performance of single vessel BHTECAB but significant challenges with multivessel BHTECAB (26).
TECAB results from mixed series
Bonaros and coworkers (27) recently published a mixed beating heart—arrested heart series of 500 TECAB cases. The procedure success rate, defined as freedom from any adverse event and freedom from conversion, was 90%. Procedure safety, defined as freedom from major adverse cardiac and cerebral events, major vascular injury, and long-term ventilation, could be determined at 95%. Predictors of success were single vessel TECAB, AHTECAB, non-learning curve case, and transthoracic assistance. Prediction of safety, however, was more related to comorbidities as defined by the EuroSCORE.
TECAB as component of hybrid coronary interventions
A hybrid coronary artery intervention is the combination of surgical coronary artery revascularization, usually performed in a less invasive way, and percutaneous coronary intervention. This concept tries to combine the best of the two worlds: the excellent long-term patency and improved survival of IMA grafting (specially to LAD target) and the minimally invasive nature of PCI. Hybrid coronary intervention represents an attractive alternative to multivessel open CABG with its inherent significant invasiveness and to multivessel percutaneous intervention with its inherent significant risk of multiple reinterventions. A considerable interest in this approach has recently been noted.
The evaluation of patients for hybrid treatment should be done by Heart Teams. That is, interventional cardiologists and robotic heart surgeons committing to work together and capable of recognizing the benefits and limitations of each of the methods. The most common scenario is a patient with multivessel disease, a complex LAD lesion and relatively simple non-LAD lesions, well amenable to PCI. The robotic team surgical performs TECAB LIMA-LAD and the interventional cardiology team carries out PCI to the non-LAD targets either days or weeks after surgery, before surgery or during the same procedure (28-30).
A multicenter international trial on the feasibility of coronary hybrid procedure published by Katz et al. in 2006 showed basic feasibility of combining robotic TECAB and PCI (29). Twenty seven patients requiring double vessel revascularization were treated with TECAB LIMA-LAD, and PCI to non-LAD targets (bare metal stents or drug-eluting stents). Three month follow-up angiography showed excellent LIMA-LAD patency at 96.3% but lower than expected PCI patency at 66.7%. No deaths or strokes were observed. One patient suffered a peri-operative MI. Early reintervention rate, primarily due to stent failures was 29.3%.
The largest series of robotically assisted hybrid coronary interventions was recently published by the senior author of this review (31). Hospital mortality was 1.3%. Early postoperative recovery was demonstrated and five year results seemed to meet standards of open coronary bypass surgery.
The timing of PCI related to TECAB in hybrid procedure was recently investigated by Srivastava and colleagues (32). The group retrospectively reviewed 238 patients submitted to hybrid treatment over a ten year period. Most patients (73%) had TECAB before PCI. Overall there was no drastic impact of timing of PCI on outcomes. But patients submitted to PCI before or at the same time as TECAB had shorter ICU and hospital LOS compared to patients who had surgery first. The authors conclude that timing of interventions can be tailored to patients need individually.
As experience has accumulated more advanced hybrid concepts are currently being explored and applied. Patients have been submitted to multivessel TECAB, BIMA use and stenting of non-LAD targets. Lee et al. described the first case of robotic totally endoscopic triple coronary bypass grafting combined with PCI in a patient with complex multivessel coronary artery disease (33).
Indications and contra-indications
Since multivessel TECAB and advanced hybrid procedure have become feasible and safe, any patient with a clinical indication for CABG can be considered for a robotic approach. Ideally the patients should be evaluated by a team of cardiologists, interventionalists and cardiac surgeons. Suitability for TECAB and for PCI can be discussed together and the options offered for the patient.
Absolute contraindications for TECAB include cardiogenic shock, hemodynamic instability and severely impaired lung function. Patients which pulmonary disease which would preclude single lung ventilation cannot be submitted to this procedure. Some other situations should be avoided in the initial experience of any group but can be managed by experienced surgeons (1): pleural adhesions, reoperation, significant space limitation (obese patients, enlarged hearts), and chest deformities. Lee and colleagues found that patients requiring conversion had significantly lower forced vital capacity and forced expiratory volume in 1 s (13).
Conversion rates are higher in the learning curve period (21% versus 7%) as reported by Schachner (34). During the learning curve low risk patients should therefore be chosen. Multimorbid patients most likely do not tolerate conversion and extensive operative times.
Regarding the use of CPB and the need to arrest the heart, some considerations are pertinent. Femoral cannulation should be performed only in the presence of large enough femoral vessels and absence of significant aortoiliac atherosclerosis. Therefore all patients should during the preoperative evaluation be submitted to a CT angiography scan of the whole aortoiliac tree. It has been shown that it is not safe to perform retrograde perfusion in patients with more than mild calcification of the aorta due to the increased risk of stroke and retrograde aortoiliac dissection. If this is the case, alternative sites of arterial cannulation can be sought, such as left axillary artery.
The use of the endoaortic occlusion balloon has its limitations. Not only a large enough femoral vessel should be present but also an ascending aortic diameter of no more than 4 cm. The balloon may not completely occlude aortas larger than that. Calcium in the ascending aorta should also be viewed as a contra-indication to the use of the balloon as its insuflation might dislodge calcium plaques, lead to embolic strokes, also the risk of balloon rupture is increased. The presence of more than mild soft atherosclerotic plaque formation on TEE scans of the thoracic aorta is an absolute contraindication for use of the endoballoon.
BHTECAB is contraindicated in patients with very small caliber coronary targets or coronary arteries which are calcified or intramyocardial. Careful preoperative assessment of coronary angiogram is the key. Intramyocardial courses of target vessels may be detected on CT angiography.
Quality of life and recovery
The main reason to pursue a less invasive approach in CABG surgery is to reduce surgical trauma, to thereby shorten recovery time, and to improve quality of life when compared to full sternotomy approaches. In an article dedicated only to quality of life comparisons Bonaros et al. (35) found that AHTECAB had shorter hospital stay and less postoperative pain than the approach through median sternotomy. At three months after operation AHTECAB patients showed better SF36 scores related to pain and physical health. Return to usual activities occurred 2-3 weeks earlier than conventional sternotomy group. Of special interest, patients converted from TECAB to sternotomy presented similar quality of life scores as primary sternotomy patients. Bonatti et al. (36) reviewing their experience in multivessel TECABG shows that mean time to return to household activities was 14 days, driving car at 21 days and performing sports at 42 days. These times are shorter than the usual time prescribed to follow sternal precautions after open CABG.
Even patients submitted to robotically assisted IMA harvesting with direct vision anastomosis through mini-thoracotomy have superior recovery times when compared with full sternotomy patients. Derose et al. (37) showed that 82% of patients submitted to this approach went back to their usual activities in 10 days after operation. Kon and colleagues (38) compared minithoracotomy CABG with off-pump full sternotomy CABG. In the less invasive approach, time to go back to all activities was 1.8 months. In the sternotomy group this was 4.4 months.
These advantages can be perfectly explained by the fact that in both TECAB and robotically assisted CABG through minithoracotomy the breastbone is completely preserved.
How to implement a TECAB program
Several robotic coronary revascularization programs failed in the past due to a lack of a stepwise approach to the operations. Robotic skills take time to mature, a long learning curve is frequently reported and the importance of simulation for training cannot be overemphasized. The training of a whole team, including tableside assistant, scrub nurses, anesthesiologists, and perfusionists will determine the success of the program. Therefore a slow and stepwise approach should be respected in developing the program. The principal surgeon has to undergo a long simulation training which includes virtual simulation, dry and wet labs, and cadaver training with the robotic system. In the Cleveland Clinic training approach the performance of the coronary anastomosis should be practiced by the trainee over 100 times in a pig heart wet lab model (39) before application in the clinical setting. The whole surgical team should simulate the procedure in a cadaver lab. Visiting an experienced center is of utmost importance to get in contact with all surgical steps.
During the initial phase of a program only part of the operation should be performed robotically. For example: LIMA harvesting done robotically and then proceeding with the rest of the operation in open fashion; robotic harvesting of the LIMA and robotic opening of the pericardium followed by LIMA to LAD anastomosis through a mini-thoracotomy; creation of LIMA-LAD anatomosis with the robot through a full sternotomy etc. Experience with remote access perfusion and endoballoon should be gained in non-robotic operations before this delicate technique is applied in robotic cases. As confidence improves complete operations can be performed.
The role of anastomotic connectors
The difficulty of performing a complex procedure, as a coronary anastomosis, in a totally endoscopic and solo fashion, led to the development of automatic anastomotic devices. The C-Port Flex A distal anastomotic device (Cardica, Redwood City, CA), launched in 2007, has a malleable shaft and is applicable through an endoscopic port (15 mm). In brief the IMA is loaded on the device. The coronary target is incised and the device “anvil” is brought into the target vessel. The table side surgeon activates the device release mechanism. The anastomosis is created by a cutting knife and application of several interrupted metal clips. Balkhy et al. (40) described 120 BHTECAB where the Flex-A device was used in either single or multivessel revascularization. Overall results include one death, one MI, three conversions to larger incisions (one sternotomy, two thoracotomies) and a mean hospital stay of 3.3 days. The short term patency rate was 94.1% for all grafts and 98.2% for LIMA-LAD.
Future directions
The last 15 years saw a great advance in the development of robotic systems and surgical techniques for TECAB. We are now using the third generation of the daVinci system which, if compared with the previous versions, allows better vision, includes a fourth arm, procedure specific instruments, and better instrument reach. An ongoing effort and interest exists in developing haptic feedback for the robotic system. Although visual clues can very well compensate for that, it seems that some form of tactile feedback could improve precision and speed in robotic tasks (41). Another line of research is the development of “virtual immobilization” that could compensate for movements of the beating of the heart, maybe facilitating the performance of off-pump operations. Single port platforms are currently developed in other fields of robotic endoscopic surgery and may have an application in robotic CABG as well.
Research in new and more effective training processes will facilitate the learning curves for new trainees. These include advances in virtual reality simulators and more effective pre-clinical training.
Interest of industry in creating new instruments and devices will be partially dictated by the diffusion and acceptance of cardiac surgeons in the actual technology. There is a need for instruments which enhance intrathoracic space and specifically the exposure of the back wall of the heart in multivessel TECAB. As use of the endoballoon is delicate some surgeons wish for development of a simple method for transthoracic cross-clamping of the aorta and infusion of cardioplegia into the aortic root from the patients left side.
Overall the advances which have been made over the first 15 years of application of robotics in coronary bypass surgery are significant. Due to the complexity of this approach, however, it will most likely not be for every surgeon. But robotic TECAB is here to stay. It is highly likely that activities will be concentrated in centers of excellence. In these centers further exciting developments can be expected.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bonatti J, Schachner T, Bonaros N, et al. Robotically assisted totally endoscopic coronary bypass surgery. Circulation 2011;124:236-44. [PubMed]
- Loulmet D, Carpentier A, d’Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [PubMed]
- Lehr EJ, Grigore A, Reicher B, et al. Dual console robotic system to teach beating heart total endoscopic coronary artery bypass grafting: a video presentation. Interact Cardiovasc Thorac Surg 2010;8:S113-4.
- Stephenson ER Jr, Sankholkar S, Ducko CT, et al. Robotically assisted microsurgery for endoscopic coronary artery bypass grafting. Ann Thorac Surg 1998;66:1064-7. [PubMed]
- Ducko CT, Stephenson ER Jr, Sankholkar S, et al. Robotically-assisted coronary artery bypass surgery: moving toward a completely endoscopic procedure. Heart Surg Forum 1999;2:29-37. [PubMed]
- Damiano RJ Jr, Ehrman WJ, Ducko CT, et al. Initial United States clinical trial of robotically assisted endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2000;119:77-82. [PubMed]
- Kappert U, Cichon R, Schneider J, et al. Closed-chest coronary artery surgery on the beating heart with the use of a robotic system. J Thorac Cardiovasc Surg 2000;120:809-11. [PubMed]
- Cichon R, Kappert U, Schneider J, et al. Robotic-enhanced arterial revascularization for multivessel coronary artery disease. Ann Thorac Surg 2000;70:1060-2. [PubMed]
- Farhat F, Aubert S, Blanc P, et al. Totally endoscopic off-pump bilateral internal thoracic artery bypass grafting. Eur J Cardiothorac Surg 2004;26:845-7. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced “robotic” cardiac surgery: experience in 148 patients. J Thorac Cardiovasc Surg 2001;121:842-53. [PubMed]
- Kappert U, Cichon R, Schneider J, et al. Technique of closed chest coronary artery surgery on the beating heart. Eur J Cardiothorac Surg 2001;20:765-9. [PubMed]
- Argenziano M, Katz M, Bonatti J, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 2006;81:1666-74; discussion 1674-5.
- Lee JD, Srivastava M, Bonatti J. History and current status of robotic totally endoscopic coronary artery bypass. Circ J 2012;76:2058-65. [PubMed]
- Bonatti J, Rehman A, Schwartz K, et al. Robotic totally endoscopic triple coronary artery bypass grafting on the arrested heart: report of the first successful clinical case. Heart Surg Forum 2010;13:E394-6. [PubMed]
- Seco M, Edelman JJ, Yan TD, et al. Systematic review of robotic-assisted, totally endoscopic coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:408-18. [PubMed]
- de Cannière D, Wimmer-Greinecker G, Cichon R, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: multicenter European experience. J Thorac Cardiovasc Surg 2007;134:710-6. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Effectiveness and safety of total endoscopic left internal mammary artery bypass graft to the left anterior descending artery. Am J Cardiol 2009;104:1684-8. [PubMed]
- Argenziano M, Katz M, Bonatti J, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 2006;81:1666-74; discussion 1674-5.
- Dogan S, Aybek T, Andressen E, et al. Totally endoscopic coronary artery bypass grafting on cardiopulmonary bypass with robotically enhanced telemanipulation: report of forty-five cases. J Thorac Cardiovasc Surg 2002;123:1125-31. [PubMed]
- Currie ME, Romsa J, Fox SA, et al. Long-term angiographic follow-up of robotic-assisted coronary artery revascularization. Ann Thorac Surg 2012;93:1426-31. [PubMed]
- Bonatti J, Lehr EJ, Schachner T, et al. Robotic total endoscopic double-vessel coronary artery bypass grafting--state of procedure development. J Thorac Cardiovasc Surg 2012;144:1061-6. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Robotic totally endoscopic double-vessel bypass grafting: a further step toward closed-chest surgical treatment of multivessel coronary artery disease. Heart Surg Forum 2007;10:E239-42. [PubMed]
- Kappert U, Cichon R, Schneider J, et al. Technique of closed chest coronary artery surgery on the beating heart. Eur J Cardiothorac Surg 2001;20:765-9. [PubMed]
- Shroyer AL, Grover FL, Hattler B, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 2009;361:1827-37. [PubMed]
- Srivastava S, Barrera R, Quismundo S. One hundred sixty-four consecutive beating heart totally endoscopic coronary artery bypass cases without intraoperative conversion. Ann Thorac Surg 2012;94:1463-8. [PubMed]
- Dhawan R, Roberts JD, Wroblewski K, et al. Multivessel beating heart robotic myocardial revascularization increases morbidity and mortality. J Thorac Cardiovasc Surg 2012;143:1056-61. [PubMed]
- Bonaros N, Schachner T, Lehr E, et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg 2013;95:803-12. [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Treatment of double vessel coronary artery disease by totally endoscopic bypass surgery and drug-eluting stent placement in one simultaneous hybrid session. Heart Surg Forum 2005;8:E284-6. [PubMed]
- Katz MR, Van Praet F, de Canniere D, et al. Integrated coronary revascularization: percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 2006;114:I473-6. [PubMed]
- Jansens JL, De Croly P, De Cannière D. Robotic hybrid procedure and triple-vessel disease. J Card Surg 2009;24:449-50. [PubMed]
- Bonatti JO, Zimrin D, Lehr EJ, et al. Hybrid coronary revascularization using robotic totally endoscopic surgery: perioperative outcomes and 5-year results. Ann Thorac Surg 2012;94:1920-6; discussion 1926.
- Srivastava MC, Vesely MR, Lee JD, et al. Robotically assisted hybrid coronary revascularization: does sequence of intervention matter? Innovations (Phila) 2013;8:177-83. [PubMed]
- Lee JD, Vesely MR, Zimrin D, et al. Advanced hybrid coronary revascularization with robotic totally endoscopic triple bypass surgery and left main percutaneous intervention. J Thorac Cardiovasc Surg 2012;144:986-7. [PubMed]
- Schachner T, Bonaros N, Wiedemann D, et al. Predictors, causes, and consequences of conversions in robotically enhanced totally endoscopic coronary artery bypass graft surgery. Ann Thorac Surg 2011;91:647-53. [PubMed]
- Bonaros N, Schachner T, Wiedemann D, et al. Quality of life improvement after robotically assisted coronary artery bypass grafting. Cardiology 2009;114:59-66. [PubMed]
- Bonatti J, Lee JD, Bonaros N, et al. Robotic totally endoscopic multivessel coronary artery bypass grafting: procedure development, challenges, results. Innovations (Phila) 2012;7:3-8. [PubMed]
- Derose JJ Jr, Balaram SK, Ro C, et al. Mid-term results and patient perceptions of robotically-assisted coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2005;4:406-11. [PubMed]
- Kon ZN, Brown EN, Tran R, et al. Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg 2008;135:367-75. [PubMed]
- Bonatti J, Alfadlhi J, Schachner T, et al. Do manual assisting maneuvers increase speed and technical performance in robotically sutured coronary bypass graft anastomoses? Surg Endosc 2007;21:1715-8. [PubMed]
- Balkhy HH, Wann LS, Krienbring D, et al. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821-7. [PubMed]
- Currie ME, Trejos AL, Rayman R, et al. Evaluating the effect of three-dimensional visualization on force application and performance time during robotics-assisted mitral valve repair. Innovations (Phila) 2013;8:199-205. [PubMed]