Extracorporeal membrane oxygenation and cytokine adsorption
Introduction
Extracorporeal membrane oxygenation (ECMO) is increasingly used for mechanical support of respiratory and cardio-circulatory failure. Veno-venous and veno-arterial modalities of ECMO are different in indications, complications and underlying pathophysiology (1). An inflammatory response is observed during the ECMO use per se and various mechanisms seem to be responsible for the activation of the inflammatory system (2). The clinical picture of systemic inflammatory response triggered by cardiopulmonary bypass (CPB) is similar to the picture as observed during sepsis. After CPB, this phenomenon is well known as CPB induced systemic inflammatory response (SIRS post-CPB) (3). Whereas sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection with a high mortality (4), the systemic inflammatory responses of sepsis and CPB have similar clinical effects (2,5). ECMO may be seen as a prolonged exposure to CPB with surface contact, initiation of inflammatory pathways and mechanical cell stress. The underlying pathophysiology of inflammatory disease may result in pulmonary, cardio-circulatory or combined disturbances which can even pronounce inflammatory overstimulation. Therefore, the therapeutic target of controlling hyperinflammation and its deleterious sequelae may be an attractive treatment approach in this context.
In this review, we describe the pathophysiological role of cytokine overstimulation and the technical aspects of cytokine adsorption as an approach to cope with excessive inflammatory response during ECMO.
Exuberant inflammation—pathophysiological aspects
Inflammatory activation occurs physiologically due to various triggers and, if uncontrolled, may result in an exuberant, overwhelming inflammatory response. This process is fueled by a release of endogenous inflammatory mediators which may lead to a systemic inflammatory response (6). An excessive systemic inflammatory reaction may have detrimental effects and cause organ damage and failure.
Sepsis is actually defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” (4) and clinical features of sepsis may be the result of an overly exuberant inflammation (7). Infection triggers a complex host response, in which pro-inflammatory and anti-inflammatory mechanisms may contribute to clearance of infection and tissue recovery, as well as to organ injury and secondary infections (8). The immune reaction depends on patient´s individual condition, coexisting diseases and the specific load and pathogenicity of the causative agent. Pro-inflammatory reactions, caused by elimination of pathogens are considered to be responsible for the collateral tissue damage, whereas anti-inflammatory immune responses, limiting the tissue injury, are considered to be responsible for the susceptibility to secondary infections (7).
The use of a CPB is well known to cause a systemic inflammatory response. This response is induced both by extrinsic and intrinsic factors. Contact activation within the extracorporeal circuit and endotoxemia represent extrinsic factors, whereas tissue damage and endothelial cell activation rank among intrinsic factors (9,10). Injured tissues react with an inflammatory response to combat the cause of injury. Multiple factors associated with the use of CPB contribute toward a systemic inflammatory response (6). Shear forces from roller pumps, hypothermia, and contact activation by artificial surfaces in the bypass circuit represent associated factors. Inflammatory responses are generated including complement, coagulation, fibrinolytic, and kallikrein cascades after exposure of blood to artificial surfaces with leukocytosis and impaired platelet function (11). These reactions may be a physiologic, temporary reaction in uncomplicated cases (10), but a systemic inflammatory response may reoccur and the postoperative course may be complicated by organ dysfunction (10). Exuberant systemic inflammatory responses in context with a CPB are also known as “post-perfusion” or “post-pump” syndrome (12). Increased capillary permeability, accumulation of interstitial fluid, and organ dysfunction result from these processes (12). Cytokines are regarded as important mediators in the systemic inflammatory response to CPB. Important pro-inflammatory cytokines in this context are interleukin (IL)-1, tumor necrosis factor (TNF), IL-6, and IL-8, which are detectable immediately postoperatively in peripheral blood (10). A release of anti-inflammatory cytokines (e.g., IL-10) may be observed at the same time (13) and balancing of dysregulated inflammatory homeostasis with increased levels of pro-inflammatory and anti-inflammatory mediators is important for the maintenance of health (13). The CPB associated systemic inflammatory response represents an unpredictable, significant risk factor of morbidity and mortality (6).
Coagulation and inflammation during ECMO
Activation of the contact system leads via factor XIIa to an increase of kallikrein and bradykinin which also drive inflammation and cause direct activation of neutrophils (2). Through several steps in the coagulation cascade prothrombin is converted to thrombin which leads to the expression of P-selectin and E-selectin by endothelial cells increasing neutrophil adherence and activation (2). Production of platelet activating factor (PAF) is also caused by thrombin and acts as another potent activator of neutrophils, which directly induce neutrophil release of pro-inflammatory cytokines (2). An activation of platelets occurs due to the conversion to thrombin and adherent platelets release their granular content, which contain various mediators, like chemokines or pro-inflammatory cytokines (14).
The inflammatory response of ECMO therapy also consists activation of the complement system (2). Activation of the endothelium plays a relevant role and occurs at an early phase in response to complement products (15). Pro-inflammatory cytokine levels increase which are responsible for the later activation of the endothelium. The infiltration of neutrophils is caused by activation of the endothelium and is considered to be responsible for the end-organ damage associated with ECMO (16).
Cytokines and cytokine storm
Host response is not only an instant local process, where leukocytes and endothelial cells are particularly involved (17). In general, infections may provoke damage not only as a consequence of virulence of pathogen. Innate immune system associated molecular patterns like pathogen- or damage-associated molecular patterns (PAMPs/DAMPs) can trigger various pathways beyond the target of defense against germs or tissue remodeling (18). The innate immune system is activated by the binding of PAMPs or DAMPs on pattern recognition receptors (PRRs), resulting in release of inflammatory cytokines (17). Cytokines are relatively small in size and represent the final step of cell signaling (17,19). In case of infection, the cytokine genes are upregulated and after fighting the infection these genes return to their normal state (19). When cytokine genes remain upregulated, the products drive into a state of permanently activated cells (19).
Cytokine release induces cytokine production and re-release, which in turn aggravates cell and organ damage (17) and may lead to an outgoing vicious circle which is also known as “cytokine storm”. The term “cytokine storm” was first used for description of a dysregulated production of inflammatory cytokines in the rapid onset of severe acute graft-versus-host-disease (20,21).
From clinical data we experienced that there is an interrelationship of cytokine response and associated clinical course: a cohort study investigated 1,886 subjects with sepsis following community acquired pneumonia and measured interleukins 6 and 10 levels (IL-6 and IL-10) (22). The highest risk of death occurred with combined high levels of the pro-inflammatory IL-6 and anti-inflammatory IL-10 cytokine activity with a hazard ratio of 20.5 (22).
The cytokine triggered overwhelming inflammatory response may lead to various compromised organ functions and therefore the approach to treat this phenomenon in theory may have a potential to mitigate disturbed organ function. So far, using convective or diffusive elimination techniques treatment modalities did not prove to efficiently solve and control cytokine storm. An adsorptive approach thus may offer a new option to cope with excessive cytokine release.
Blood purification and cytokine removal
Blood purification methods were used to reduce cytokine levels during exuberant inflammatory responses in septic patients and they seem to be promising techniques for stabilization of hemodynamics and oxygenation (23). The model behind blood purification techniques consists in the “cytokinetic model”: this means increasing the cytokine concentration gradient from plasma to infected tissue by removing inflammatory mediators from the blood (23). This reconstitution of cytokine gradients is followed by leukocyte trafficking to the infectious focus, resulting in increased local bacterial clearance (23).
So far, blood purification consisted of dialytic techniques including high-volume hemofiltration (HVHF), high adsorption hemofiltration, high cut-off membrane hemofiltration, plasma exchange, and hybrid systems like coupled plasma filtration adsorption (24). HVHF increases plasma water exchange and the mainly water-soluble mediators seem to be removed in a relevant manner from the plasma and hemofilters may have additional adsorptive properties (23). Most inflammatory mediators rank among the middle-molecular-weight molecules (from 5 to 60 kDa) and are mainly removed by membrane adsorption, followed by convection which is more effective than diffusion in removing middle molecules (25).
Convection, diffusion, and adsorption are different mechanisms for blood purification and some data exist of effective removal of inflammatory mediators, but data of timing, duration, and frequency in the clinical setting are still lacking in the literature and large randomized controlled trials are still needed (23).
The recent international guidelines for management of sepsis and septic shock made no recommendation for blood purification because of very low confidence in the evidence and declared that further research is needed to clarify the benefit of various blood purification techniques (26).
Mechanisms of hemoadsorption with Cytosorb®
The extracorporeal cytokine hemoadsorption device Cytosorb® (Cytosorbents Corporation, USA) has been designed to directly capture and reduce mid-molecular weight inflammatory mediators (~10–60 kDa). Pro- and anti-inflammatory cytokines, chemokines, and bacterial exotoxins are affected (27,28). The Cytosorb® hemoadsorption device was approved in Europe in 2011 and can actually be used as a stand-alone therapy or in combination with extracorporeal circuits like continuous renal replacement therapy (CRRT), CPB and ECMO. The technology is based on highly porous, biocompatible nonpolar polymer beads that are capable of capturing inflammatory mediators through size exclusion and non-specific surface adsorption throughout the entire bead. The technology of polymer bead-based cytokine hemoadsorption has shown to rapidly eliminate cytokines in vitro and in vivo (23,29). Cartridges contain divinylbenzene copolymer beads with biocompatible coating from which the beads are 300–800 µm in size (30). Each 300 mL cartridge is filled with polymeric sorbent beads, achieving an effective surface area of more than 40,000 m2. The substances are adsorbed due to physiochemical binding depending on the concentration. Therefore, the efficacy for elimination is most prominent for substances at high concentrations, while significantly less effective on substances at low concentrations.
So far, over 23,000 treatments have been performed in more than 500 clinical departments around the world. No harmful removal of immune cells as leukocytes or hemolysis of red blood cell has been reported (3,5,31-33). Albumin plasma levels were reported to be reduced less than 5% (33) and plasma levels of platelets lowered less than 10% (33), which may be associated due to mechanism of the extracorporeal circuit per se. To our knowledge, no clinical relevant adverse effect has been reported for hemoadsorption treatment with Cytosorb® so far.
Cytokine adsorption and vascular barrier function
IL-6 is known to be linked to the trans-signaling pathway, which causes vascular leakage (34). Capillary leakage is followed by tissue edema, hypoxia and finally necrosis. Thus, the highly increased IL-6 level is a valuable target for therapeutic intervention to terminate capillary leakage. Another important aspect of capillary leakage is related to the endothelial glycocalix. The integrity of the endothelial glycocalix is essential for endothelial barrier function (35). Strategies to preserve intact glycocalix are needed to reduce vascular leakage (36). A positive effect of cytokine removal with hemoadsorption therapy (Cytosorb®) on the integrity of vascular barrier function was described in a case report of a septic patient (37). Adsorptive elimination of high-level pro-inflammatory cytokines like IL-6 may be a major target to prevent loss of vascular barrier function, capillary leakage and consecutively may diminish organ damage.
Blood purification and mortality
A meta-analysis of blood purification in septic patients showed that the blood purification treatment group had a mortality of 35.7% compared to 50.1% in the conventional treatment group (24). Blood purification techniques decreased mortality in patients with sepsis, severe sepsis or septic shock. These pooled results of multiple small randomized studies of moderate quality demonstrated that blood purification (including hemoperfusion or plasma exchange alone, hemofiltration combined with hemoperfusion) is associated with lower mortality in patients with sepsis. Low-quality evidence indicates that blood purification with continuous veno-venous hemofiltration (CVVH) might be associated with reduction in mortality in patients with sepsis or acute respiratory distress syndrome (ARDS) (38).
Hemoadsorption and cytokine removal in septic patients
In a descriptive case series of 26 critically ill patients with septic shock and high grade acute kidney injury (AKI) necessitating renal replacement therapy (post-surgical and medical) were treated with a combined application of CVVHD and Cytosorb® (39). Patient outcome was analyzed in relation to time of treatment start. The continuous venovenous hemodialysis (CVVHD)/Cytosorb® treatment was associated with a pronounced decrease in catecholamine demand, stabilization of hemodynamics and a decrease in blood lactate in the overall patient population (39). All ICU and hospital survivors in this case series had been treated early (<24 h) after onset of septic shock, whereas none of the patients who was treated after 48 h after onset of septic shock survived. From these data starting the hemoadsorption early (less than 24 h after onset of septic shock) together with sepsis bundle measures appeared to be advantageous in terms of survival (39).
An observational case series in 16 cardiac surgical patients following prolonged CPB with massive post-bypass systemic inflammatory response with highly elevated cytokine levels and acute kidney failure described a reduction of the elevated cytokine levels, stabilization of hemodynamics and organ function after hemoadsorption with Cytosorb® combined with CVVHD (3).
A case series on 39 cardiac surgical patients with infective endocarditis who were treated with hemoadsorption (Cytosorb®) during CPB described balanced control of the inflammatory response postoperatively with reduced cytokine levels, rapid normalization of lactate, base excess, and hemodynamics (5).
The first randomized study investigating potential preventive effects of hemoadsorption with Cytosorb® studied 32 patients undergoing elective CPB surgery. This study investigated the course of pro-inflammatory cytokine levels after Cytosorb® treatment during CPB (32). As a result, there were no differences between the hemoadsorption and the control group, but a longer-lasting anti-inflammatory effect of IL-10 in the hemoadsorption group was observed (32). Nevertheless, this was the first randomized, controlled study for hemoadsorption therapy in cardiac surgical patients. Therefore, under these conditions pre-emptive hemoadsorption therapy for elective CPB obviously did not have any major impact on clinical patient course.
A case of a 9-month-old infant reported the successful use of hemoadsorption therapy with Cytosorb® in combination with continuous hemodiafiltration due to sepsis after cardiac surgery with multi organ failure (40). A relevant decrease of catecholamine doses, as well as aminotransferase plasma levels and bilirubin (total and direct) plasma levels was observed. Hemoadsorption therapy can also reduce other endogenous and exogenous compounds due to its physicochemical mechanism.
ECMO, hemoadsorption and cytokine removal
So far mainly anecdotal reports are available about the use of Cytosorb® hemoadsorption during ECMO treatment.
Bruenger et al. reported about a 39 years old patient with ARDS and septic cardiogenic failure treated with hemoadsorption and ECMO (27). The hemoadsorption was installed into the CVVH circuit. The patient was initially treated with veno-arterial extracorporeal membrane oxygenation (VA-ECMO), followed by a left ventricular assist device and a right heart extra corporeal life support. During three sessions of hemoadsorption over four days the plasma levels of IL-6 and procalcitonin (PCT) decreased and the vasopressor need could be significantly reduced (27).
In a recent case report a 64 years old patient with a post infarction ventricular septum defect had to be treated with a VA-ECMO due to severe shock with multi organ failure refractory to conventional hemodynamic therapy (41). The patient underwent 4 days of combined VA-ECMO and hemoadsorption therapy and vasopressors could be reduced and metabolic conditions normalized (41).
Lees et al. reported combined treatment with VA-ECMO and hemoadsorption in a 33 years old patient suffering from acute left ventricular failure due to sepsis. The hemoadsorption was installed into the CVVH circuit for 24 hours. Initially high dose vasopressors could be weaned off after 24 hours and the patient remained asymptomatic in the follow-up two month later (42).
We treated a 45 years old patient with severe ARDS with VA-ECMO and combined hemoadsorption therapy (28). An overwhelming inflammatory response with excessively increased cytokines was present with concomitant severe multi organ failure. A total of three successive treatments with a combination of Cytosorb® and CVVHD over 85 hours was performed. As result a marked decrease in IL-6 and IL-8 plasma levels could be observed concomitant with a decline of capillary leakage, hemodynamic stabilization, and respiratory improvement (28).
All these case reports share the clinical pictures of detrimental shock with pronounced hyper-inflammatory disease treated with combined ECMO and hemoadsorption. The most obvious clinical pattern consists of reduced cytokine levels accompanied by hemodynamic stabilization and reduced vasoactive medication. So far, no controlled, prospective and randomized study is available about the combined use of ECMO and hemoadsorption.
ECMO and hemoadsorption—technical aspects and limitations
The need of an extracorporeal circuit for implementation of hemoadsorption therapy has various aspects and limitations which must be kept in mind for clinical use. Every added connection in an extracorporeal circuit can lead to accidental air embolism, contamination, and at least disconnection.
Most patients treated with hemoadsorption devices receive various medication like antibiotics and the adsorptive drug elimination must be considered. Therefore, further investigations are warranted to clarify the influence of hemoadsorption therapy on drug elimination.
So far, clear recommendations and sound evidence of hemoadsorption therapy are lacking regarding timing, duration and dosing and therefore further investigations are needed.
The hemoadsorption device in most described cases was installed into the CRRT circuit when it was combined with an ECMO device. The reported cases were mainly courses of critically ill patients with multi organ failure and need for renal replacement therapy. The hemoadsorption cartridge could easily implemented inline into the CRRT circuit due to low blood flow rates during CRRT. In general, the cartridge may be also installed in the ECMO circuit. The limited blood flow allowed through the cartridge necessitates the integration in a parallel flow setup. It must be kept in mind, that the hemoadsorption cartridge like Cytosorb® is not approved for high flow circuits with continuous flow rates of several liters per minute like ECMO devices. Of note, hemoadsorption therapy with Cytosorb® is recommended by the manufacturer for limited flows up to 700 mL/min. Apart from independent hemoadsorption circuits, possible direct implementations of the hemoadsorption cartridge are presented in Figures 1,2 for passive and active settings. Nevertheless, approved connections are absolutely needed for the respective used ECMO system and available for some common used systems.
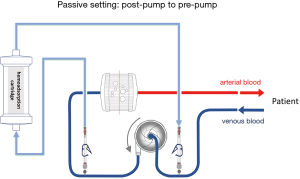
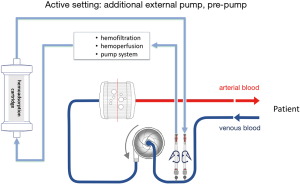
So far, commonly used ECMO circuits do not offer standardized manufacturer-proofed connective ports to integrate a hemoadsorptive device. It has to be considered that accidental disconnection of a high flow circuit may have deleterious effects and needs to be avoided. Generally applicable tubing systems with special connections and with regard to these safety issues are under development and should be recently commercially available.
Cytokine elimination with hemoadsorption—actual state for clinical use
So far, most data of hemoadsorption therapies are available for patients with septic shock. Data for hemoadsorption therapy in combination with ECMO were only reported individual cases. It has to be considered that hemoadsorption therapy and other blood purification techniques are still without sound recommendation for clinical use in the actual guidelines for management of sepsis and septic shock because of lacking high class evidence. Nevertheless, cytokine adsorption seems to offer a promising new treatment approach for patients with severe cardiocirculatory failure and excessive inflammatory response. Current data also indicate that hemoadsorption may exert an impact on endothelial glycocalix and therefore it might be advantageous for maintenance of the vascular barrier function.
In conclusion, the answer to the question is still open, if and how cytokine adsorption may impact the clinical course in the most critical scenarios with septic shock and organ support with ECMO and if this therapeutic approach may finally influence patient outcome. This treatment option, however, could add a new therapeutic approach that has to prove its role in the treatment bundle of our sickest patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: K Träger received honoraria for lectures from Cytosorbents and has an advisory contract with Cytosorbents.
References
- Clark JB, Wang S, Palanzo DA, et al. Current Techniques and Outcomes in Extracorporeal Life Support. Artif Organs 2015;39:926-30. [Crossref] [PubMed]
- Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016;20:387. [Crossref] [PubMed]
- Trager K, Fritzler D, Fischer G, et al. Treatment of post-cardiopulmonary bypass SIRS by hemoadsorption: a case series. Int J Artif Organs 2016;39:141-6. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Trager K, Skrabal C, Fischer G, et al. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass - a case series. Int J Artif Organs 2017;40:240-9. [Crossref] [PubMed]
- Day JR, Taylor KM. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005;3:129-40. [Crossref] [PubMed]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840-51. [Crossref] [PubMed]
- van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis 2008;8:32-43. [Crossref] [PubMed]
- Tomic V, Russwurm S, Moller E, et al. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation 2005;112:2912-20. [PubMed]
- Chew MS, Brandslund I, Brix-Christensen V, et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology 2001;94:745-53; discussion 5A.
- Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1993;55:552-9. [Crossref] [PubMed]
- Chenoweth DE, Cooper SW, Hugli TE, et al. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med 1981;304:497-503. [Crossref] [PubMed]
- McBride WT, McBride SJ. The balance of pro- and anti-inflammatory cytokines in cardiac surgery. Curr Opin Anaesthesiol 1998;11:15-22. [Crossref] [PubMed]
- Whiteheart SW. Platelet granules: surprise packages. Blood 2011;118:1190-1. [Crossref] [PubMed]
- Fischetti F, Tedesco F. Cross-talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity 2006;39:417-28. [Crossref] [PubMed]
- Mc IRB, Timpa JG, Kurundkar AR, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest 2010;90:128-39. [Crossref] [PubMed]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017;39:517-28. [Crossref] [PubMed]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469-78. [Crossref] [PubMed]
- Dinarello CA. Historical insights into cytokines. Eur J Immunol 2007;37 Suppl 1:S34-45. [Crossref] [PubMed]
- Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood 1992;80:2964-8. [PubMed]
- Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc 1993;25:1216-7. [PubMed]
- Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 2007;167:1655-63. [Crossref] [PubMed]
- Rimmele T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care 2011;15:205. [Crossref] [PubMed]
- Zhou F, Peng Z, Murugan R, et al. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med 2013;41:2209-20. [Crossref] [PubMed]
- De Vriese AS, Vanholder RC, Pascual M, et al. Can inflammatory cytokines be removed efficiently by continuous renal replacement therapies? Intensive Care Med 1999;25:903-10. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Bruenger F, Kizner L, Weile J, et al. First successful combination of ECMO with cytokine removal therapy in cardiogenic septic shock: a case report. Int J Artif Organs 2015;38:113-6. [Crossref] [PubMed]
- Trager K, Schutz C, Fischer G, et al. Cytokine reduction in the setting of an ARDS-Associated inflammatory response with multiple organ failure. Case Rep Crit Care 2016;2016:9852073. [Crossref] [PubMed]
- Kellum JA, Song M, Venkataraman R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med 2004;32:801-5. [Crossref] [PubMed]
- Song M, Winchester J, Albright RL, et al. Cytokine removal with a novel adsorbent polymer. Blood Purif 2004;22:428-34. [Crossref] [PubMed]
- Hetz H, Berger R, Recknagel P, et al. Septic shock secondary to beta-hemolytic streptococcus-induced necrotizing fasciitis treated with a novel cytokine adsorption therapy. Int J Artif Organs 2014;37:422-6. [Crossref] [PubMed]
- Bernardi MH, Rinoesl H, Dragosits K, et al. Effect of hemoadsorption during cardiopulmonary bypass surgery - a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care 2016;20:96. [Crossref] [PubMed]
- Schädler D, Porzelius C, Jörres A, et al. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Critical Care 2013;17:62.
- Kruttgen A, Rose-John S. Interleukin-6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res 2012;32:60-5. [Crossref] [PubMed]
- Noble MI, Drake-Holland AJ, Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM 2008;101:513-8. [Crossref] [PubMed]
- Henrich M, Gruss M, Weigand MA. Sepsis-induced degradation of endothelial glycocalix. ScientificWorldJournal 2010;10:917-23. [Crossref] [PubMed]
- David S, Thamm K, Schmidt BMW, et al. Effect of extracorporeal cytokine removal on vascular barrier function in a septic shock patient. J Intensive Care 2017;5:12. [Crossref] [PubMed]
- Putzu A, Fang MX, Boscolo Berto M, et al. Blood purification with continuous veno-venous hemofiltration in patients with sepsis or ARDS: a systematic review and meta-analysis. Minerva Anestesiol 2017;83:867-77. [PubMed]
- Kogelmann K, Jarczak D, Scheller M, et al. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care 2017;21:74. [Crossref] [PubMed]
- Cirstoveanu CG, Barascu I, Mc Kenzie Stancu S. Hemadsorption with Adult CytoSorb(R) in a Low Weight Pediatric Case. Case Rep Crit Care 2017;2017:6987167. [Crossref] [PubMed]
- Marek S, Gamper G, Reining G, et al. ECMO and cytokine removal for bridging to surgery in a patient with ischemic ventricular septal defect - a case report. Int J Artif Organs 2017;40:526-9. [Crossref] [PubMed]
- Lees NJ, Rosenberg A, Hurtado-Doce AI, et al. Combination of ECMO and cytokine adsorption therapy for severe sepsis with cardiogenic shock and ARDS due to Panton-Valentine leukocidin-positive Staphylococcus aureus pneumonia and H1N1. J Artif Organs 2016;19:399-402. [Crossref] [PubMed]


