Aortic valve replacement through J-shaped partial upper sternotomy
Introduction
Aortic valve replacement (AVR) has been performed through a standard median sternotomy for over 40 years. The minimally invasive revolution affecting general surgery in the late 1980s also influenced cardiac surgery resulting in the emergence of several minimal access approaches for AVR. The parasternal approach to AVR was the first such approach reported by Cosgrove and Sabik in 1996 (1). In 1997 Bennetti and colleagues (2) described the right thoracotomy approach. This was followed by the description in 1998 by Gundry and colleagues (3) of the partial ministernotomy approach for both adult and pediatric cases. A transverse sternotomy approach was also briefly utilized but quickly abandoned due to unacceptable morbidity and mortality rates (4). Currently the two most popular approaches are the upper ministernotomy and the right thoracotomy approach (5).
The upper partial sternotomy with unilateral J-shaped extension to the right through the fourth intercostal space provides a window through which the aortic root is freely accessible (6,7). Cardiopulmonary bypass is established through this incision and no new instruments, retractors, and ports are necessary. With only modest increase in difficulty and without additional risk to the patients the surgeon utilizes familiar techniques and the patient benefits from prompt recovery provided the patients are properly selected (7). This article provides a comprehensive review of the indications, contraindications, technical aspects, outcomes, advantages and disadvantages of AVR through J-shaped partial upper sternotomy.
Indications & contraindications
J-shaped partial upper sternotomy is best suited for isolated AVR and aortic root replacement in patients of all ages. The technique is particularly applicable to the elderly and those with impaired respiratory function since both pleural cavities can be kept intact (7). The level of the sternal division necessary to provide access to the aortic annulus varies greatly with body habitus, the presence or absence of chronic obstructive airway disease, and whether the heart lies transversely or longitudinally within the chest. The standard preoperative chest radiography provides valuable information about the relative positions of the ascending aorta, its root and the sternum. In some cases one can even recognise the exact level of the aortic valve because of its calcifications (7). For all practical purposes we recommend extension to the right through the fourth intercostal space for good exposure of the right atrium as contrary to general perception it is access to right atrium and not the aortic root which determines the need for femoral venous cannulation.
Presence of significant coronary artery disease and porcelain aorta are absolute contraindications for this approach. Relative contraindications include very short or very long ascending aorta, poor ejection fraction, small aortic root in elderly patients requiring patch enlargement, and last but not the least lack of availability of transesophageal echocardiography.
Technique
Prior to transfer to operating theatre, a triple lumen central venous line, a radial artery catheter, Swan-Ganz catheter with the provision to pace the heart and external defibrillation paddles are placed. A single lumen endotracheal tube is used. The patient is prepared and draped accordingly to provide access to the whole length of the sternum with the groins and upper legs prepared as for all valve operations. A straight skin incision of approximately 2.5 to 3 inches (7 to 8 cm) is then made from the level of the head of the second rib in the midline over the sternum and extended down to the level of the head of the fourth rib (Figure 1A). The skin and subcutaneous tissue are undermined up to the sternal notch and, at the lower extent, into the fourth intercostal space. Either a regular (pendulum) saw or an oscillating saw can be used, but particularly for reoperations, it is essential to use an oscillating saw to open the sternum because of adhesions. The right internal thoracic artery (ITA) is usually 1 cm away from the sternal edge and can be protected by placing a forceps around the sternal edge to push the right ITA laterally away from the saw.
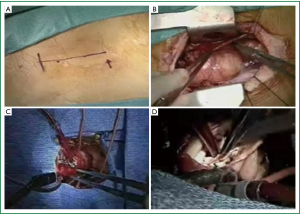
Although the right pleural cavity can be preserved but we preferentially open this cavity as it enables drainage of pericardial cavity into the pleural space. Additionally, the pericardial drain and pacing wires traverse the right pleural space to reach the pericardial cavity upon completion of surgery precluding the need to bring these accessories below the xiphisternum, a cumbersome and mostly blind undertaking.
A Finocchietto retractor is inserted and the mediastinal tissues exposed (Figure 1B). Thymic tissue is then dissected and excised if necessary providing the usual access to the upper anterior pericardium. Keeping to the mid line the pericardium is then opened from the innominate vein to beneath the lower intact sternal table. Critical to good exposure is the use of multiple, heavy silk sutures on the pericardium to pull the aorta and right atrium into good view (Figure 1B). This may result in ventricular diastolic filling dysfunction and increased right-sided pressures, but these are reversed by release of the sutures after cardiopulmonary bypass. The aorta is then cannulated just proximal to the innominate artery in a conventional manner and a two stage venous cannula inserted into the right atrial appendage (Figure 1C). For enhanced exposure of the aortic root we place a silk tie around the venous cannula and pull it laterally by bringing out this silk tie through a hole in chest wall that is used later for the chest tube and pacing wires insertion. The mode of cardioplegic arrest depends on surgeon’s preference. Direct anterograde delivery of cold crystalloid or blood cardioplegia is simple but a retrograde cannula can also be placed lower in the right atrium either blindly or with transesophageal echocardiographic guidance. A standard angled or curved aortic cross clamp is applied and sits out of the surgical field if well placed (Figure 1C). From this point the aortic procedure does not deviate from normal until the de-airing stages. Valve or full root replacement or repair is accomplished according to surgeon’s preference. The surgical field is kept dry by a suction vent in either the left superior pulmonary vein (our preferred choice), main pulmonary artery or the bottom of the left ventricle (trans-aortic) all of which are easily accessible with this method.
For stentless AVR a transverse incision 0.5 cm above the aortic sinuses and at least 1 cm above the right coronary ostium is preferable (7). For mechanical valve implantation and use of stented bioprostheses we prefer the standard oblique incision extending down to the annulus in the non-coronary sinus. For aortic valve procedures, exposure of the aortic valve is aided by placing commissure sutures under tension (Figure 1D).
With the new valve reliably implanted it is important to secure closure of the aortotomy since bleeding from the root is less easily accessible via this approach when the heart is full. The de-airing process must also be thorough since this is achieved predominantly through the highest point of the aorta. Left ventricular vent suction is discontinued as the aortotomy is closed so that the heart fills. The patient is placed in a head down position and rhythmic inflation of the lungs helps to expel air into the left ventricular outflow tract. A DLP® aortic root cannula (Medtronic Inc., USA) with suction vent on the highest point of the aorta helps to evacuate air bubbles. Partial aortic cross-clamping distally to the suction vent can improve the de-airing process. If the heart does not spontaneously defibrillate then internal paediatric sized paddles are applied to the epicardium.
Transesophageal echocardiography is used continuously to check de-airing and to detect right ventricular disfunction due to air embolism which may require a period of continued support. It is useful to place the right ventricular pacing wires before the aortic cross clamp is released as bleeding from placement of pacing wires can be quite challenging to manage with the heart full and beating.
After successful weaning of cardiopulmonary bypass the cannulas are clamped and removed. Protamine is administered. The suture lines, cannulation sites and other potential sites are checked for bleeding and then the pericardial stay sutures are released. The sternal edges are accurately opposed with steel wires taking care not to damage the internal thoracic arteries. With the J-incision four wires between the two halves of the sternal table are sufficient. An additional wire for the horizontal limb of the J-incision ensures sternal stability.
Outcomes
Ministernotmy AVR versus full sternotomy
At the authors’ institution since 2003 to 2013, a total of 928 patients have undergone isolated first time surgical AVR. AVR through J-shaped partial upper sternotomy has been performed in 167 patients and AVR through full sternotomy in 761 cases. Preoperative characteristics are summarized in Table 1. Cohort receiving full sternotomy AVR had older patients, reduced (<50%) or poor (<30%) left ventricular function, diabetics on insulin, non-elective surgical indication and a previous myocardial infarction compared to ministernotomy cohort. Contrary to published literature, in our experience cross clamp time tended to be shorter in the ministernotomy group, with no significant difference in cardiopulmonary bypass time. The mean size of implanted prosthesis did not differ among the two groups.
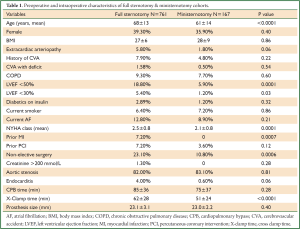
Full Table
Operative outcomes are shown in Table 2. Ministernotomy AVR was associated with a trend towards better operative outcomes including need for re-intubation, postoperative need for intra-aortic balloon pump and lower 30-day mortality. Ministernotomy AVR was significantly associated with a shorter length of hospital stay.
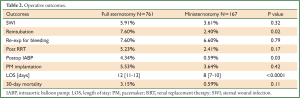
Full Table
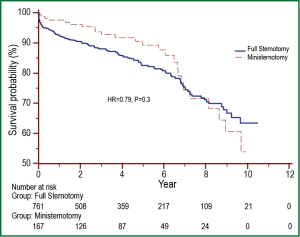
After a mean follow-up time of 4.0±2.9 years (range, 0.09-10.4 years), late survival was comparable among ministerontomy and full sternotomy AVR groups (P=0.3) as shown in Figure 2.
Sharony et al. (8) published a propensity score analysis comparing 233 patients with ministernotomy AVR to 233 patients undergoing full sternotomy AVR. Hospital mortality and major morbidity were similar in the ministernotomy and full sernotomy groups: 5.6% versus 7.3% (P=0.45) and 13.3% versus 14.2% (P=0.79), respectively. Multivariable analysis of all patients revealed increased mortality with severe atheromatous aortic disease (P=0.001), chronic obstructive pulmonary disease (P=0.002), and urgent operation (P=0.02). Freedom from any major perioperative morbidity was similar in both groups (86.7% versus 85.8%; P=0.79). However, the median length of stay was shorter with ministernotomy AVR (6 versus 8 days; P<0.001).
Similar outcomes have been reported by several other authors (9-15).
Reoperative ministernotomy AVR
Tabata et al. (16) have published the largest experience to date of J-shaped ministernotomy approach for reoperative AVR. This approach minimizes mediastinal dissection and handling of friable tissues on sternal reentry, resulting in reduced transfusion requirement and decreased operative time relative to a full sternotomy (17). For patients with patent grafts, this approach minimizes the risk of graft injury. Left ITA grafts are not dissected, and other aortocoronary grafts are exposed only at their proximal part, which is usually identified without difficulty (18).
From July 1996 to June 2007 at Brigham and Women’s hospital, 146 patients underwent reoperative minimal access aortic valve surgery, 109 of whom had undergone previous coronary artery bypass grafting and 93 of whom had a patent left ITA graft (16). Median age was 76 years, and 43 patients (29%) were 80 years or older. Nineteen patients (13%) underwent concomitant procedures, such as coronary artery bypass grafting, mitral valve repair, and ascending aortic replacement. Median cardiopulmonary bypass and aortic cross clamp times were 150 and 80 minutes, respectively. Four patients (2.8%) had conversion to full sternotomy. Operative mortality was 4.1% (6/146). The incidences of reoperation for bleeding and blood transfusion were 0.7% (1/146) and 83.6% (122/146), respectively. No patient had left ITA or aortocoronary graft injury. Median stay was 8 days, and 56% (79/140) were discharged home. Five-year actuarial survival was 85%.
This study confirms that J-shaped ministernotomy approach is safe and feasible in reoperative aortic valve surgery, with excellent early and late outcomes.
Ministernotomy AVR with minimal extracorporeal circulation (MECC)
Until recently most of the cardiac surgical operations have been performed with conventional cardiopulmonary bypass (CPB). Many studies have demonstrated the deleterious effects of CPB such as hemodilution and cytokine response, and initiating coagulation cascade (19,20). To overcome these drawbacks of CPB, Wiesenack and colleagues (21) presented a retrospective series of patients undergoing coronary artery bypass surgery with MECC demonstrating reduction in postoperative complications and blood loss. Since then many studies have demonstrated the beneficial effects of MECC in lowering the postoperative inflammatory responses (22,23). In addition, AVR by standard median sternotomy utilizing MECC has been shown to have better outcomes than AVR with conventional perfusion techniques (24).
Yilmaz and associates (25) reported outcomes for 50 consecutive patients (24 males) with a mean age of 68 (range, 34 to 89) years that underwent ministernotomy AVR utilizing MECC. Operating time was 147±20 minutes, cross-clamp time was 64±10 minutes, and perfusion time was 84±17 minutes. There were no conversions to median sternotomy. Only one peroperative blood transfusion was required and postoperative blood loss was 372±170 cc. Intensive care unit stay was uneventful (average stay 2 days, range 1 to 8 days). One patient required a permanent pacemaker and other complications included pneumothorax, superficial wound infection, a late transient postoperative neurologic deficit, and excessive postoperative blood loss requiring mediastinal reexploration. Renal failure and major cerebral accidents did not occur. There was a 100% survival at one-month follow-up. This study validates the effectiveness of J-shaped partial upper sternotomy approach in combination with MECC for AVR.
Ministernotomy approach for sutureless AVR
Santarpino et al. (26) recently published their experience of sutureless aortic valve implantation via ministernotomy. Seventy-two patients (43 women, 29 men; mean age 77.4±5.3 years) with isolated aortic valve stenosis (mean gradient of 52±14 mmHg) underwent aortic valve implantation with the sutureless Perceval S bioprosthesis (Sorin Group, Saluggia, Italy) using standard CPB techniques. The prosthetic valve was successfully deployed in all patients. Thirty-day mortality was 1.4% (n = 1). Mean CPB, aortic cross clamp, and implantation times were 68±18, 40±13, and 8.9±4 min, respectively. Perioperative echocardiography revealed significant paravalvular leakage in one patient. Postoperative mean gradient was 11.6±5.1 mmHg. At a mean follow-up of 13±6.7 months, no significant paravalvular leakage or valvular regurgitation was observed, and no migration or dislodgement of the prosthesis occurred. This study confirms the safety and efficacy of combining ministernotomy approach with sutureless valve technology.
Folliguet and associates (27) have also reported similar outcomes for their cohort of 45 patients that underwent sutureless AVR through ministernotomy using Perceval S bioprosthesis (Sorin Group, Saluggia, Italy).
Advantages & disadvantages
The J-shaped upper partial sternotomy offers the comfort factor of sternotomy over thoracotomy and prevents complications secondary to distentions at the costovertebral joint or brachial plexus traction at the thoracic inlet (7). Additional well-established benefits include better preservation of thoracic respiratory mechanics, superior cosmetic result, early mobilization, reduced length of stay, ease of conversion to full sternotomy, and performance of surgery without the need for new equipment.
On the other hand, real as well as perceived disadvantages including inability to see the whole heart, inadequate de-airing, difficulty with placement of epicardial pacing wires, need for femoral cannulation, iatrogenic injury to the internal thoracic arteries, limited control in case of hemorrhage, and a steep learning curve are some of the reasons that preclude universal adoption of this technique.
Conclusions
Surgical AVR through J-shaped partial upper sternotomy is a safe and effective strategy. It can be the procedure of choice for all primary isolated aortic valve operations barring presence of porcelain aorta. This approach can be used preferentially for elderly patients, combined with MECC, and is an attractive option for implantation of sutureless aortic valves. Good cosmetic result, stable chest wall integrity, preserved respiratory mechanics after the operation with reduced patient discomfort and pain, no need for new equipment, and ease of conversion to full sternotomy in an emergency situation make this approach a “must have” in the armamentarium of modern day cardiac surgeon.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996;62:596-7. [PubMed]
- Benetti FJ, Mariani MA, Rizzardi JL, et al. Minimally invasive aortic valve replacement. J Thorac Cardiovasc Surg 1997;113:806-7. [PubMed]
- Gundry SR, Shattuck OH, Razzouk AJ, et al. Facile minimally invasive cardiac surgery via ministernotomy. Ann Thorac Surg 1998;65:1100-4. [PubMed]
- Bridgewater B, Steyn RS, Ray S, et al. Minimally invasive aortic valve replacement through a transverse sternotomy: a word of caution. Heart 1998;79:605-7. [PubMed]
- Kim BS, Soltesz EG, Cohn LH. Minimally invasive approaches to aortic valve surgery: Brigham experience. Semin Thorac Cardiovasc Surg 2006;18:148-53. [PubMed]
- Svensson LG, D’Agostino RS. J” incision minimal-access valve operations. Ann Thorac Surg 1998;66:1110-2. [PubMed]
- von Segesser LK, Westaby S, Pomar J, et al. Less invasive aortic valve surgery: rationale and technique. Eur J Cardiothorac Surg 1999;15:781-5. [PubMed]
- Sharony R, Grossi EA, Saunders PC, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. J Heart Valve Dis 2004;13:887-93. [PubMed]
- Masiello P, Coscioni E, Panza A, et al. Surgical results of aortic valve replacement via partial upper sternotomy: comparison with median sternotomy. Cardiovasc Surg 2002;10:333-8. [PubMed]
- Liu J, Sidiropoulos A, Konertz W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. Eur J Cardiothorac Surg 1999;16 Suppl 2:S80-3. [PubMed]
- Mahesh B, Navaratnarajah M, Mensah K, et al. Mini-sternotomy aortic valve replacement: is it safe and effective? Comparison with standard techniques. J Heart Valve Dis 2011;20:650-6. [PubMed]
- Corbi P, Rahmati M, Donal E, et al. Prospective comparison of minimally invasive and standard techniques for aortic valve replacement: initial experience in the first hundred patients. J Card Surg 2003;18:133-9. [PubMed]
- Detter C, Deuse T, Boehm DH, et al. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thorac Cardiovasc Surg 2002;50:337-41. [PubMed]
- Doll N, Borger MA, Hain J, et al. Minimal access aortic valve replacement: effects on morbidity and resource utilization. Ann Thorac Surg 2002;74:S1318-22. [PubMed]
- Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81:1599-604. [PubMed]
- Tabata M, Khalpey Z, Shekar PS, et al. Reoperative minimal access aortic valve surgery: minimal mediastinal dissection and minimal injury risk. J Thorac Cardiovasc Surg 2008;136:1564-8. [PubMed]
- Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg 1999;118:991-7. [PubMed]
- Byrne JG, Karavas AN, Adams DH, et al. Partial upper re-sternotomy for aortic valve replacement or re-replacement after previous cardiac surgery. Eur J Cardiothorac Surg 2000;18:282-6. [PubMed]
- Raja SG, Dreyfus GD. Modulation of systemic inflammatory response after cardiac surgery. Asian Cardiovasc Thorac Ann 2005;13:382-95. [PubMed]
- Raja SG, Berg GA. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Card Surg 2007;22:445-55. [PubMed]
- Wiesenack C, Liebold A, Philipp A, et al. Four years’ experience with a miniaturized extracorporeal circulation system and its influence on clinical outcome. Artif Organs 2004;28:1082-8. [PubMed]
- Bical OM, Fromes Y, Gaillard D, et al. Comparison of the inflammatory response between miniaturized and standard CPB circuits in aortic valve surgery. Eur J Cardiothorac Surg 2006;29:699-702. [PubMed]
- Biancari F, Rimpiläinen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009;95:964-9. [PubMed]
- Remadi JP, Rakotoarivello Z, Marticho P, et al. Aortic valve replacement with the minimal extracorporeal circulation (Jostra MECC System) versus standard cardiopulmonary bypass: a randomized prospective trial. J Thorac Cardiovasc Surg 2004;128:436-41. [PubMed]
- Yilmaz A, Rehman A, Sonker U, et al. Minimal access aortic valve replacement using a minimal extracorporeal circulatory system. Ann Thorac Surg 2009;87:720-5. [PubMed]
- Santarpino G, Pfeiffer S, Sirch J, et al. Minimally invasive aortic valve replacement with Perceval valves: first clinical experience. J Cardiovasc Med (Hagerstown) 2013. [Epub ahead of print]. [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [PubMed]


