Post-transplant native pneumonectomy for interstitial fibrosis and small cell lung cancer
Introduction
Pulmonary resection may occasionally be required after lung transplant. We describe the management of a patient who previously underwent a single lung transplant and subsequently developed extensive infectious changes and small cell lung carcinoma (SCLC) of the native lung. Written consent was obtained from the patient for publication of this case report and all accompanying images.
Case presentation
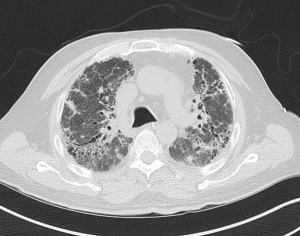
A 70-year-old Hispanic man with previous 30-pack-year smoking history presented with a chronic cough. Sixty-four months prior, the patient underwent a right single lung transplant with concurrent single vessel coronary artery bypass graft for end-stage idiopathic pulmonary fibrosis (IPF) and coronary artery disease. Figure 1 shows diffuse infiltrative fibrosis of both lungs. The patient was a cytomegalovirus (CMV) seropositive recipient of a CMV seropositive donor. Maintenance immunosuppression with prednisone, tacrolimus, and azathioprine was used per our institution’s transplantation protocol. Voriconazole, valganciclovir, and sulfamethoxazole-trimethoprim were used for opportunistic infection prophylaxis.
At 3 months post-transplant, the patient had a transient decline in pulmonary function. Bronchoscopy with transbronchial biopsies and respiratory cultures revealed Pseudomonas aeruginosa, and the patient was treated with ciprofloxacin. Azathioprine was discontinued 9 months after transplantation. Pulmonary function test steadily improved over the next 2 years, from a baseline predicted forced expiratory volume-one second percent (FEV1%) of 55% to 81%.
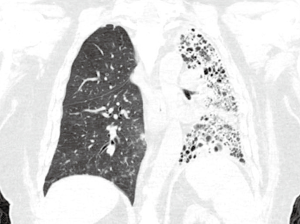
Beginning 3 years after transplantation, the patient developed a chronic cough, occasionally productive of green sputum. Over the next 2 years, he required several hospitalizations with administration of intravenous antibiotics for chronic relapsing P. aeruginosa and Escherichia coli bronchitis. At 64 months post-transplant, he presented with a worsening, productive cough and subjective fevers. His FEV1% had declined to 46% and computed tomography (CT) of the thorax revealed a diffuse infiltrative and fibrotic process of the native left lung (Figure 2). No other imaging studies were felt to be necessary.
The patient subsequently underwent a left pneumonectomy with intercostal muscle flap buttressing of the bronchial stump. Estimated blood loss was 300 milliliters. His surgery and postoperative course were uncomplicated. He was discharged to home in good condition on postoperative day 5. His chronic cough was eliminated by the time of discharge.
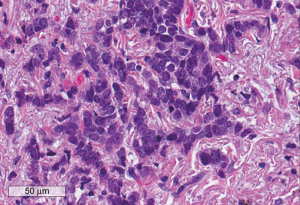
Pathological examination showed diffuse end stage lung changes and bronchiectasis. In addition, an occult, poorly differentiated 1.2-centimeter focus of small cell carcinoma was identified in the apical region of the lung specimen (Figure 3). All 15 lymph nodes were negative for malignancy. No vascular or perineural invasion was identified. After discussion with the oncology teams, he was referred for adjuvant chemotherapy.
The patient experiences a significant improvement in functionality over the course of the next several months. At 6 months after pneumonectomy, he was able to perform all household activities, could walk outside for prolonged periods of time and had no oxygen requirement.
Discussion
IPF is a chronic, progressive form of lung disease characterized by fibrosis of unknown cause. Management strategies include supportive care with supplemental oxygen and pulmonary rehabilitation, and medical therapies such as pirfenidone and nintedanib. Lung transplantation is the only known definitive treatment. It can be an excellent option for patients with progressive disease and limited comorbidities. We report a very rare case of native pneumonectomy for diffuse pneumonia, IPF and early stage SCLC approximately 5 years after single-lung transplantation for IPF.
Post-lung transplant malignancy is usually identified histologically as non-small cell lung carcinoma (NSCLC) (1,2); SCLC is a rare finding and has been described in only a handful of cases (3,4). Lung cancer screening in the high-risk post-transplant patient population is important, as the rate of lung cancer in these patients is almost 20 times higher than the rate in the general population (5). All previous reported cases of post-transplant SCLC were noted to have suspicious findings on screening chest CT. We agree that yearly chest CT should be performed for post-transplant patients to detect lung cancer at an earlier, potentially resectable stage. Our case is unique in that the chest CT did not detect an obvious focus of lung cancer, as the patient’s extensive fibrosis and end-stage lung changes obscured any potential nodules. Our patient’s diagnosis was made incidentally after left pneumonectomy for IPF and diffuse pneumonia.
Though pneumonectomy for control of symptoms in a post-transplant patient is very unusual, we felt that surgery was warranted in our patient because the entire left lung was diseased. Given the extensive anatomic damage and infiltration of the native lung, we were unable to render it microbe-free without resection. In select cases, surgical resection of damaged and infected native lung may provide an improved quality of life for post-transplant patients.
SCLC is characterized by a relatively rapid rate of growth and spread compared to NSCLC, and is usually not detected at an early stage. Even though only 2–5% of SCLC patients present with stage I disease (6), our patient was able to have his disease resected at an early stage because it was found incidentally. It is likely that by the time our patient developed local or systemic symptoms related to SCLC, there would have been a significantly greater tumor burden present.
Per the National Comprehensive Cancer Network guidelines, adjuvant chemotherapy is recommended in patients after surgical resection of stage I SCLC to decrease the risk of recurrence (7). However, treatment strategies in the post-transplant setting are poorly defined in the literature. Du et al. (8) presented a similar case of stage I SCLC found in the transplanted lung. That patient underwent radiation therapy with 50 Gy and chemotherapy with four cycles of carboplatin and etoposide. Few conclusions can be drawn from this paucity of cases in the literature, however, and more cases of SCLC in the post-transplant setting need to be reported before treatment plans can be optimized for this unique patient population.
In patients with chronic obstructive pulmonary disease (COPD) and IPF who are candidates for lung transplantation, single vs. double-lung transplantation is an important decision, especially given the scarcity of lung donors. Post-transplant malignancy more commonly affects the native lung versus the donor lung (1,2). In addition, when comparing single-lung vs. double-lung transplant, the incidence of lung cancer is markedly higher in single-lung transplantation (6.9–9.8%) when compared to double-lung transplantation (0–1.8%). Though the risk of post-transplant malignancy should be considered when evaluating patients for lung transplant, the overall risk is very low and multiple other clinical factors should be used as well to make the decision of single versus double-lung transplant.
Chronic immunosuppression is a significant risk factor for developing malignancy. In the literature, some have advocated for decreased dosages or discontinuation of azathioprine and/or calcineurin inhibitors. Mammalian target of rapamycin (mTOR) inhibitors have demonstrated immunosuppression and antitumor benefits with some published benefits in management of post-kidney transplant Kaposi’s sarcoma, but its role in lung transplant patients is not well studied.
This interesting case report presents a rare case of early stage SCLC found after native lung pneumonectomy, 64 months after single-lung transplantation. Though the indication for pneumonectomy was chronic suppuration, the patient also benefitted from resection of his SCLC. Native pneumonectomy can be performed safely and treat patients affected by infectious and/or malignant disease processes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Olland AB, Falcoz PE, Santelmo N, et al. Primary lung cancer in lung transplant recipients. Ann Thorac Surg 2014;98:362-71. [Crossref] [PubMed]
- Van Raemdonck D, Vos R, Yserbyt J, et al. Lung cancer: a rare indication for, but frequent complication after lung transplantation. J Thorac Dis 2016;8:S915-24. [Crossref] [PubMed]
- Moniodis A, Racila E, Divo M. Case report: combined small cell lung cancer in a lung transplant recipient. Transplant Proc 2015;47:852-4. [Crossref] [PubMed]
- De Soyza AG, Dark JH, Parums DV, et al. Donor-acquired small cell lung cancer following pulmonary transplantation. Chest 2001;120:1030-1. [Crossref] [PubMed]
- Stagner LD, Allenspach LL, Hogan KK, et al. Bronchogenic carcinoma in lung transplant recipients. J Heart Lung Transplant 2001;20:908-11. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs 2007;67:1167-98. [Crossref] [PubMed]
- Du L, Pennell NA, Elson P, et al. Lung cancer treatment outcomes in recipients of lung transplant. Transl Lung Cancer Res 2015;4:784-91. [PubMed]


