Routine aortic valve replacement followed by a myriad of complications: role of 3D printing in a difficult cardiac surgical case
Introduction
Aortic stenosis (AS) due to calcification is the most frequent valve disease in America and Europe (1). While aortic valve replacement (AVR) is the definitive therapy of severe AS, the advanced age of the affected patients poses increased operative risk. The overall mortality of the procedure is 1–3%, with infection, excessive blood loss, and thromboembolism being the most common complications (2). Here we present a complex case with some of the most severe postoperative complications following AVR.
Case presentation
A 55-year-old female patient underwent AVR with xenograft implantation (first operation) due to AS, who had no prior apparent coronary artery disease. Following cardiac surgery, the patient was readmitted to our clinic owing to inferior ST-segment elevation myocardial infarction (STEMI). Coronary arteriography showed right coronary artery (RCA) occlusion that necessitated an urgent cardiac surgery with application of intra-aortic balloon pump (IABP, second operation). During the intervention, type A aortic dissection was observed which occluded the right coronary and had to be bypassed with a saphenous vein graft (SVG). However, the reconnection of the left main (LM) was only possible with a Gore-Tex interpositum because of the malignant adhesions. Furthermore the procedure was converted into deep hypothermic circulatory arrest (DHCA) in order to perform open distal anastomosis to the arch. So we ended up with a modified Bentall procedure, whereas the right coronary was bypassed with SVG.
Following surgery, the patient was transferred to intensive care unit (ICU), where postoperative transesophageal echocardiography (TEE) examination revealed deterioration of right ventricular function that was attributable to the previous RCA infarction, hence right ventricular assist device (RVAD) was implanted immediately (third operation).
The right ventricle recovered following 26 days of mechanical circulatory support (MCS), and the patient was successfully weaned from the device. During this (fourth) chest opening, the sternum got infected and negative pressure wound therapy (NPWT) was necessary. Since the sternum has broken apart, we decided to close the chest with laminar sternum-osteosynthesis. Six months later, control computed tomography (CT) angiography described RCA graft occlusion and significant discrepancy of the interpositum-LM diameters. One year later, our patient returned with Canadian Cardiovascular Society (CCS) class III angina and critical stenosis of the LM-graft anastomosis together with a giant aortic pseudoaneurysm revealed on control CT (Figure 1).
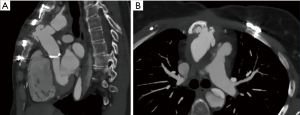
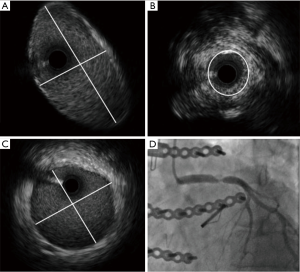
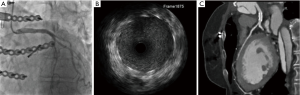
Since a repeated surgical revascularization bore immense perioperative risk due to aortic pseudoaneurysm and titanium osteosynthesis, the team decided to perform LM-PCI, while the reconstruction of the pseudoaneurysm was postponed. Angiographic evaluation showed a short, non-bifurcating lesion, with a diameter of 6 mm (Figure 2). After reference vascular diameter verification with intravascular ultrasound (IVUS) (Figure 2) and balloon pre-dilation (4.0 mm × 20 mm), a 5.0 mm × 15 mm cobalt-chromium drug eluting stent was implanted (Figure 3). Optimization was done with a 6.0 mm × 20 mm peripheral balloon and controlled with IVUS. One month later, CT control verified a well conducting stent (Figure 3).
During the rehabilitation, the patient started to feel her pulse between the first and second rib. Echocardiography revealed rapid extrathoracic dilation and perfusion of the aortic pseudoaneurysm.
Following urgent readmission of the patient, the accurate extension of the pseudoaneurysm could not be characterized by routine CT imaging due to the implanted titanium lamina, as it produced an extended artefact, making the separation impossible. Therefore, using CT angiogram images—three-dimensional (3D) model of the aortic pseudoaneurysm was reconstructed with self-developed MATLAB script and the planned stl file was printed in 3D (Figure 4). As a result, the border zone between titanium lamina and the wall of pseudoaneurysm became precisely separable on the printed 3D model (3) (Figure 4). During the fifth operation (Figure 5), this model was the basis for the accurate localization of the aneurysmatic bulging and separation of titanium lamina from the aortic pseudoaneurysm sac. After axillo-femoral cannulation, we started to cool down the patient, during which we removed the titanium osteosynthetic plate carefully (Figure 5), since 3D CT reconstruction images showed its adhesion with the aortic pseudoaneurysm. After successful removal of the titanium plates, we opened the chest with an oscillating saw. However, the aortic pseudoaneurysm ruptured as was anticipated (Figure 6), but the patient’s body temperature was 16 Celsius degree and cardiopulmonary bypass was already applied. We have explored the lesion, where the 4-0 Prolene was fiberized and ruptured, and the grafto-graftal conduit anastomosis was opened. The anastomosis was reconstructed and we started to warm up the patient after 38 minutes of DHCA. We were able to successfully wean the patient from bypass and close the chest with ZipFix. The patient was extubated on the third day and admitted for rehabilitation 12 days after cardiac surgery, who is currently symptomless.
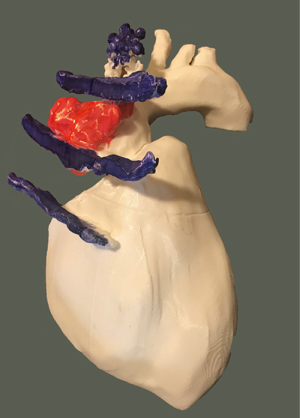
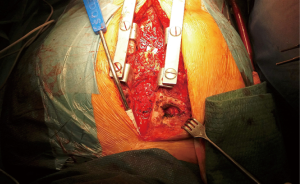
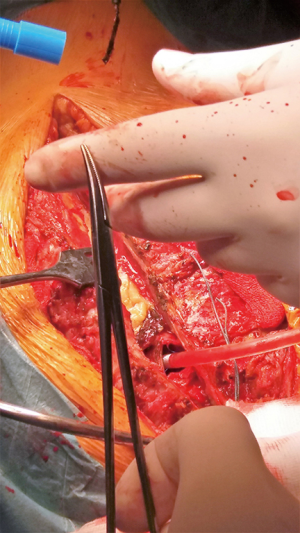
Discussion
We presented a case of frequent cardiac disease with unique complications following routine cardiac surgery. We also show the emerging importance of 3D printing technique in chest surgery (4).
Acknowledgements
The applied MATLAB script and 3D planning of the aortic aneurysm was accomplished by Imre János Barabás.
Funding: This study was supported by the National Research, Development and Innovation Office of Hungary (NKFIH; NVKP-16-1-2016-0017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The study participant provided informed consent for the findings of this case to be published.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Chen N, Zhu K, Zhang H, et al. Three-dimensional printing guided precise surgery for right-sided aortic arch associated with Kommerell's diverticulum. J Thorac Dis 2017;9:1639-43. [Crossref] [PubMed]
- Yuan D, Luo H, Yang H, et al. Precise treatment of aortic aneurysm by three-dimensional printing and simulation before endovascular intervention. Sci Rep 2017;7:795. [Crossref] [PubMed]


