Should the ART trial change our practice?
Background
Acute respiratory distress syndrome (ARDS) is one of the most severe forms of acute hypoxemic respiratory failure. Caused by pulmonary or systemic insults, is characterized clinically by hypoxemia that does not respond to the administration of high concentrations of oxygen (FiO2) and by the presence of bilateral infiltrates on chest imaging due to high-permeability pulmonary edema (1). An integral part of the supportive therapy of ARDS is the application of mechanical ventilation (MV). The goal of MV is to achieve adequate gas exchange and tissue oxygenation without further damaging the lungs. Since the first description of ARDS in 1967 (2), the use of positive end-expiratory pressure (PEEP) has been adopted as standard practice for its ventilatory management. PEEP prevents end-expiratory alveolar collapse. The pivotal ARDSnet trial published in 2000 (3) demonstrated that a “lung-protective” MV strategy using a tidal volume (VT) of 4–8 mL/kg of predicted body weight (PBW) and moderate levels of PEEP improved survival. Since then, limitation of VT to less than 8 mL/kg PBW and plateau pressure to less than 30 cmH2O, and application of PEEP between 10 to 20 cmH2O represents the standard for MV in ARDS.
Today, most patients with ARDS improve their oxygenation (as assessed by the PaO2/FiO2 ratio) after the application of moderate to high levels of PEEP. When defining ARDS, the specific ranges and conditions to evaluate the PaO2/FiO2 ratio have varied considerably. The American-European Consensus Committee (4) and the Berlin criteria (5) proved to be incapable of identifying uniform groups of patients in terms of severity and outcome since there are no data that link a particular baseline PaO2/FiO2 to predictable structural changes in the alveolar-capillary membrane at the time of ARDS onset. However, there is evidence showing a correlation between lung injury severity and outcome when PaO2/FiO2 is assessed under standard ventilatory settings at 24 hours after ARDS onset (6,7).
In a high proportion of ARDS patients, severe hypoxemia persists beyond the first 24 hours. Classic computed tomography (CT) has shown that some lung regions in ARDS appear radiographically to be relatively normal, whereas some other areas are partially collapsed and unable to participate in gas exchange (8). Collapsed or atelectatic areas of the lung can be re-expanded by the application of a brief period of high transpulmonary pressure followed by the application of adequate levels of PEEP to maintain the new aerated regions open (9). These recruitment maneuvers (RMs) are intended to reopen collapsed alveoli and to ameliorate the injurious effects of repetitive opening and closing of lung units and tidal overdistension by restoring the functional size of the lung, promoting lung protection, improving gas exchange and lung mechanics (10). However, the primary factor for the sustained improved oxygenation is the level of PEEP after the RM. Because PEEP is an expiratory setting, its level should be tailored after having recruited the lung, that is identifying the lowest PEEP level sustaining the recruited lung open. This is the theoretical basis for the decremental PEEP trial (9-12).
Today more controversy exists over the benefits of RMs in persistent ARDS than in any other aspect of ventilatory management of ARDS. In a pilot randomized controlled trial (RCT) in 200 ARDS patients with persistent hypoxemia comparing the ARDSnet protocol (3) with an open lung approach (OLA)—which involved RMs and a decremental PEEP trial identifying the PEEP level associated with the maximum dynamic compliance, Kacmarek et al. (12) reported that OLA improved oxygenation and respiratory system mechanics without detrimental effects on 60-day mortality, ventilator-free days, or barotrauma. This trial identified the need for a larger RCT using RMs in association with PEEP titrated by compliance of the respiratory system to test whether this approach is able to increase survival in patients with persistent ARDS. Such a trial, known as the Alveolar Recruitment for ARDS trial (ART), has been published recently (13) and it constitutes the basis for this editorial. Based on their findings, the authors concluded that the use of their OLA increased 28-day mortality in patients with moderate-to-severe ARDS, suggesting that the routine use of lung RM and PEEP titration cannot be recommended in persistent ARDS. However, a careful and critical review of the study identifies more questions than answers, due to several problems and weaknesses in the study design and methodology.
Concerns and sources of bias in ART
As stated in the ART paper (13), the trial was conducted in 120 ICUs in nine countries between November 2011 and April 2017. A total of 1,010 adult patients with moderate-to-severe ARDS of <72 hours’ duration were enrolled. At 28 days, 49.3% (251/509) of patients ventilated with a low-PEEP strategy (control group) and 55.3% (277/501) of patients in the OLA (experimental) group had died. The authors stated that the 28-day and 6-month mortalities were significantly different between the two groups. Analyses of secondary outcome variables showed that there were no significant differences in the length of ICU and hospital stay or in the rates of ICU or hospital mortality between the two groups. However, there are several aspects and limitations that critically question the acceptance and generalizability of the results and conclusions of this RCT.
First, since the implementation of lung protective MV, the overall ICU mortality of ARDS has consistently remained below 45% in all observational studies (14,15). It is very surprising and inexplicable that the figures for 28-day, ICU, hospital, and 6-month mortalities reported in the ART trial are all above any reported figures for patients ventilated with lung protective MV enrolled in all RCTs comparing different ventilatory modalities and adjunctive approaches since 1990 (3,12,16-26) (Table 1). Specifically, since the establishment of the ARDSnet protocol as the management approach for control groups, none of the recent RCTs had a 28-day mortality above 39%, and 60- or 90-day mortalities were all equal to or less than 45% (12,14,16-26).
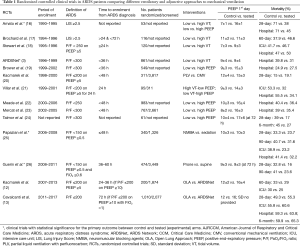
Full table
The question then is why the high mortality? The ART trial was essentially a Brazilian study, accounting for 104 of the 120 participating ICUs. Demographic, cultural, economic, and health-care system differences with USA, Canada, Australia, and Western European countries could partially explain the excessive mortality in the ART trial. Although Brazil has many highly skilled tertiary hospitals, the World Health Organization ranks its health care system 125 out of 190 countries (27). Given the large number of participating ICUs, regional differences in the general health status of the population, bed utilization, hospital and ICU staffing, scarcity in ICU resources, and burden of diseases requiring ICU admission, may all have adversely contributed to overall patient outcome. Regarding the latter aspect, it is remarkable that were virtually no exclusion criteria to study entry related to previous patient morbid conditions or number of organ failures, both critically influencing ICU outcome. All the above considerations make the generalizability of the results of the ART trial highly questionable.
Also, of concern is whether individual centers acquired the required skills and proper training to implement and efficiently conduct such a complex clinical protocol. It is not clear how quality of performance was assured and controlled in all 120 participating ICUs which very likely had large differences in standards of care. Anyone who has been involved in RCTs understands that communication, discussion and constant review and monitoring of problems and issues associated with the conduction of the study are essential for the consistent application of complex interventional protocols. In our recent OLA trial (12), the vast majority of investigators met every 6 months and a newsletter was regularly sent to investigators discussing study issues and problems. It is our understanding that the ARDSnet investigators met on a monthly basis. Thus, and based on our experience, we question how the adoption and execution of a 112-page detailed very complex protocol with implementation difficulties continually occurring was effectively communicated to all 120 centers? How was it assured that centers understood and applied the experimental protocol consistently and properly? Again, in our OLA RCT (12) participating centers were required to perform pilot studies with discussion of the most difficult aspects of the protocol and potential problems before individual centers were allowed to begin randomization. In spite of these quality control measures, we found many protocol violations across centers when analyzing our results (12). In contrast, surprisingly only limited protocol violations were reported in the ART study; there were reports regarding the performance of the RMs but no other protocol violations in either group were discussed.
Second, the P values for the statistical difference of all-cause 28-day and 6-month mortalities reported in the paper using Kaplan-Meier curves and the calculated hazard ratio with 95% CI using the Cox proportional hazard model are correct. We reanalyzed and tested the 28-day and 6-month mortality rates using the Fisher’s exact test and chi-squared test, and found that the differences were not statistically different (P=0.059 for 28-day and P=0.079 for 6-month, two-sided). Even when computing the relative risk (RR) and 95% confidence intervals (CI) for 28-day mortality in the experimental group, we did not find significant differences: RR 1.12, 95% CI: 0.99–1.26, P=0.058). Thus, based on these analyses, the ART study did not show that RM plus decremental PEEP trial were inferior to the low-PEEP control group.
Third, patients were recruited if they had a PaO2/FiO2 ≤200, provided they were not ventilated for longer than 72 hours. Before confirming eligibility, patients were evaluated under a standardized ventilator setting using PEEP ≥10 and FiO2 =1 for 30 min. Only patients with a persistent PaO2/FiO2 ≤200 were eligible for randomization. This approach differs from previously published studies using standardized ventilatory settings (6,12). Under FiO2 of 1.0, the effects of ventilation/perfusion mismatch are eliminated and true shunt is measured (28), but ventilation with 100% oxygen induces absorption atelectasis and increases true shunt unless adequate PEEP is applied. Villar et al. (6) assessed PaO2/FiO2 ratio in 170 patients with moderate-to-severe ARDS ventilated with lung protective MV under two levels of PEEP (≥5 and ≥10 cmH2O) and two levels of FiO2 (≥0.5 and 1) at two time-periods (ARDS onset and 24 h later). They found that the setting that best identified patients with persistent moderate-to-severe ARDS and predicted differences in ICU mortality were PEEP≥10 on FiO2 ≥0.5 at 24 hours after ARDS onset. They also found that assessment under FiO2 of 1.0 with PEEP≥10—the method used in the ART trial—did not identify patients stratified by severity of illness.
Fourth, the protocol for lung recruitment required excessive pressure and time in all patients randomized to the OLA arm. In an attempt to compensate for the acidosis developed during the lengthy RM, the ART protocol required the respiratory rate be increased to 35/min for 20 min preceding the RM. Peak recruiting pressure in the RM arm was mandated at 60 cmH2O. Driving pressure set at 15 cmH2O, then PEEP was increased in one step to 25 cmH2O held for 1 min, then to 35 cmH2O held for 1 min and then to 45 cmH2O and held for 2 min. The decremental PEEP trial began at 23 cmH2O and ended at 11 cmH2O with pressure decreased in 3 cmH2O steps but maintained at each step for 4 min. After the decremental PEEP trial, PEEP was increased in one step from 11 to 45 cmH2O to reestablish the peak pressure of 60 cmH2O and held for 2 min. After the second RM, PEEP was set at the best compliance PEEP plus 2 cmH2O determined during the detrimental PEEP trial. This was very similar to the overall method used in the OLA trial (12) except that PEEP during recruitment was slowly increased in small PEEP steps to the peak recruiting pressure which in the vast majority of RMs was 50 cmH2O and held for 1 min. In fact, in only 18 patients (10 at 55 cmH2O and 8 at 60 cmH2O), was the RM considered necessary at a peak pressure above 50 cmH2O. The decremental PEEP trial began at 25 cmH2O. After a 3-min stabilization period, PEEP was decreased in 2 cm H2O steps until the best compliance PEEP could be identified. However, each step was only held until the dynamic compliance stabilized, usually 30 to 60. The RM after the decremental PEEP was performed the same as the initial RM. Thus, the ART total recruitment process took about 24 min while ours took only 10–12 min depending on the best compliance PEEP. Of major concern with the ART trial was that 3 patients suffered a cardiac arrest and 7 developed pneumothoraces during the recruitment process and the minimal PEEP in the open lung group was 13 cmH2O. Since the decremental PEEP trial was limited to 11 cmH2O, excessive PEEP may have been applied to some patients. Cardiac arrest and pneumothoraces during the RM never occurred in the OLA trial (12) nor have any of us experienced them clinically nor have they been reported in the literature (29). These findings highlight the problems with the protocol, the lack of experience of the investigators and proper training of individuals applying the protocol.
After randomization of the first 555 patients, the protocol was changed limiting peak recruiting pressure to 50 cmH2O and the time for PEEP steps was decreased to 3 min. In the RM arm of the ART trial a total of 44 pneumothoraces were reported vs. 14 in the low PEEP group. In the OLA study (12), there were six pneumothoraces in the OLA arm but none developed during a RM while in the ARDSnet arm eight pneumothoraces were reported. In the OLA trial, RMs and titrated PEEP significantly improved oxygenation and driving pressure when compared to the ARDSnet protocol without detrimental effects on mortality and ventilator-free days. Paradoxically, a recent RCT performed in patients with hypoxemia after cardiac surgery admitted to a single ICU in Brazil examining the effects of RM added to protective MV, reported that the use of RM (45 cmH2O peak pressure/30 cmH2O PEEP, repeated 3 times) resulted in less severe pulmonary complications (30).
Fifth, the ventilatory settings for the lung recruitment arm are a concern. In this arm, patients were ventilated in volume/assist control until weaning when pressure support was applied. However, all patients were ventilated with a VT <6 mL/kg PBW with a square wave flow pattern, 60 liters/min peak flow, a 0.5 s inspiratory pause and a respiratory rate of 35/min unless pH >7.45. As indicated by the ART authors in the supplemental material, double triggering and breath stacking was very likely a common occurrence and we presume based on our own experiences, flow asynchrony was also common. As recently shown by Yoshida et al. (31), a strong ventilator drive coupled with a small VT in ARDS causes marked pendelluft increasing the likelihood of ventilator-induced lung injury (VILI) despite small delivered VT. In the OLA trial (12), we attempted to adjust ventilation to the specific needs of each patient. We used pressure assist/control in the OLA arm and maintained VT between 4 to 8 mL/kg PBW with VT, inspiratory time and respiratory rate adjusted to meet the patients’ neural inspiratory time and ventilatory demand. Based on our results, we believe that individual patient adjusted settings reduced the likelihood of VILI. Thus, simply by the design of the lung recruitment strategy and the approach to ventilation, we assume that the likelihood of VILI was very high in patients in the ART trial randomized to the recruitment arm, accounting at least partially for the high mortality in this group.
The future!
In summary, concerns with the study design, methodology, data analyses, and results—in addition to possible major differences with health care systems—provide solid arguments to question the results of the ART trial and the advisability of generalizing its results to other settings. We believe there is still a strong pathophysiological rationale for the use of RM and decremental PEEP trial in moderate-to-severe ARDS, supporting the principle that “never give the lung a chance to collapse”. Unfortunately, the ART study forces us to reassess the use of RMs and decremental PEEP trials since the results of the ART trial conflict with previously acquired data. The results of this study have not dampened our enthusiasm for the OLA but have identified the need for another RCT that is designed and implemented in a manner that will more appropriately test the ability of the OLA to improve outcome in ARDS (9,10,12).
Acknowledgements
Funding: This work was supported in part by Instituto de Salud Carlos III, Madrid, Spain (PI13/0119, PI16/00049, CB06/06/1088) and Asociación Científica Pulmón y Ventilación Mecánica.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Villar J. What is the acute respiratory distress syndrome? Respir Care 2011;56:1539-45. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Villar J, Pérez-Méndez L, López J, et al. An early PEEP/FiO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;176:795-804. [Crossref] [PubMed]
- Villar J, Blanco J, del Campo R, et al. Assessment of PaO2/FiO2 for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open 2015;5:e006812. [Crossref] [PubMed]
- Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med 2005;31:776-84. [Crossref] [PubMed]
- Girgis K, Hamed H, Khater Y, Kacmarek RM. A decremental PEEP trial identifies the PEEP level than maintains oxygenation after lung recruitment. Respir Care 2006;51:1132-9. [PubMed]
- Borges JB, Okamoto VN, Matos GF, et al. Reversibility of lung collapse and hypoxemia in early respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:268-78. [Crossref] [PubMed]
- Suárez-Sipmann F, Böhm SH, Tusman G, et al. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med 2007;35:214-21. [Crossref] [PubMed]
- Kacmarek RM, Villar J, Sulemanji D, et al. Open lung approach for the acute respiratory distress syndrome. Crit Care Med 2016;44:32-42. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 2016;22:1-6. [Crossref] [PubMed]
- Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009;179:220-7. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;158:1831-8. [Crossref] [PubMed]
- Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med 1998;338:355-61. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Kacmarek RM, Wiedemann HP, Lavin PT, et al. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;173:882-9. [Crossref] [PubMed]
- Villar J, Kacmarek RM, Pérez-Méndez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311-8. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome. A randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Mercat A, Richard JCM, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome. A randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- World Health Organization’s Ranking of the World’s Health Systems. Available online: , accessed 16 October 2017.http://thepatientfactor.com/canadian-health-care-information/world-health-organizations-ranking-of-the-worlds-health-systems/
- Shapiro BA, Crane RD, Harrison RA, et al. Changes in intrapulmonary shunting with administration of 100 percent oxygen. Chest 1980;77:138-41. [Crossref] [PubMed]
- Fan E, Wilcox ME, Brower RG, et al. Recruitment maneuvers for acute lung injury: a systematic review. Am J Respir Crit Care Med 2008;178:1156-63. [Crossref] [PubMed]
- Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung protective ventilation on postoperative pulmonary complications. A randomized clinical trial. JAMA 2017;317:1422-32. [Crossref] [PubMed]
- Yoshida T, Nakahashi S, Nakamura MAM, et al. Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. Am J Resp Crit Care Med 2017;196:590-601. [Crossref] [PubMed]


