The pathological and molecular diagnosis of malignant pleural mesothelioma: a literature review
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor that arises from mesothelial cells lining the pleural cavity. Its occurrence is related to exposure to mineral fibers, particularly asbestos, which represents the main risk for MPM, with a latency period of approximately 40 years between fiber exposure and disease presentation (1,2).
MPM diagnosis continues to be difficult and should utilize morphological assessment, correlated with the appropriate clinical and radiologic contexts and supplemented by ancillary diagnostic techniques, particularly immunohistochemistry (IHC) and, more recently, molecular tests. The pathological approach to pleural lesions should always be based on the results obtained from adequate biopsies (less commonly cytology) in terms of both tissue quality and quantity. Since the key indicator of malignancy remains the invasion of pre-existing tissue, multiple (a minimum of five biopsies is recommended), large and deep pleural biopsies comprising soft tissue of the parietal pleural tissue or lung are necessary. In this context, thoracoscopy is considered the preferred biopsy technique (3-5).
In the present article, we review some aspects concerning the pathological diagnostic approach of MPM, underlining the use of immunohistochemical and molecular markers. Particularly, we present the cytological features of malignancy and the histological patterns of MPM. Moreover, we focus on two main diagnostic problems: the differential diagnosis of benign and malignant mesothelial proliferations and the differential diagnosis of MPM and other malignant pleural tumors.
Data sources
For this review article, a PubMed search (http://www.ncbi.nlm.nih.gov/pubmed), updated August 15, 2017, was performed, combining the terms MPM and diagnosis. A total of 3,809 articles were found; only English language publications were considered. The most relevant articles selected as the basis for this review on MPM were detailed prospective studies in large series, evidence-based guidelines and papers on very specific areas, such as immunohistochemical and molecular markers with outlooks on the future. Reference materials also included textbooks.
Cytological diagnosis of MPM
A common symptom of mesothelioma is represented by recurrent pleural effusions, which are routinely submitted for cytological examination (smears and/or cell blocks). Since on cytology it is impossible to assess invasion by neoplastic mesothelial cells into sub-pleural tissue or lung parenchyma, establishing a definitive diagnosis of MPM by cytological examination alone remains controversial, especially in the light of the medico-legal implications correlated with the diagnosis of MPM (6,7). However, in selected cases in which more invasive procedures are contraindicated, the cytological diagnosis of MPM, which relies on a different set of both cellular and architectural features and is supported by ancillary techniques, can be performed, although its sensitivity is low compared to histology. In fact, the published sensitivity of cytology for the diagnosis of mesothelioma ranges from 30% to 75% (8-11). Moreover, in the cases in which histology is not available, a close correlation with clinical and imaging findings is essential for a definitive diagnosis.
Not all MPMs exfoliate tumor cells in the pleural cavity; particularly, the malignant cells in sarcomatoid mesothelioma tend not to be shed into the effusion fluid. Thus, the effusion could only contain reactive epithelioid mesothelial cells, and these cells may mislead the pathologist. In these cases, a core biopsy (or larger specimens) is necessary to establish a definitive diagnosis, particularly when surgery is considered, because the presence of a sarcomatoid component may influence therapeutic management (12).
There are several cytological features in pleural effusions that raise varying levels of suspicion for MPM, such as the extent of mesothelial proliferation, the presence of papillary structures, scalloped borders of cell clumps, intercellular windows, variation of cytoplasmic staining and its density, and low nuclear-to-cytoplasmic ratios (Figure 1). However, some of the cytomorphological findings of MPM are shared between reactive and malignant epithelioid mesothelial cells. Therefore, the differential diagnosis of mesothelial proliferations may be very difficult or even impossible in cytological specimens, underscoring the importance of ancillary techniques to clarify diagnosis (6,7).
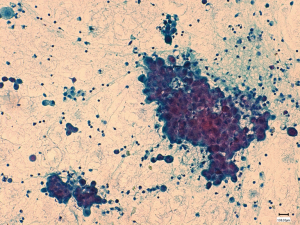
The application of immunocytochemistry (ICC) and molecular methods, such as fluorescent in situ hybridization (FISH) performed preferentially on cell blocks, increases the diagnostic accuracy of cytology (13-16). The differential diagnosis of MPM and the use of ICC and molecular markers in cytological samples are the same as in histological specimens. Several ICC markers, such as desmin, p53, epithelial membrane antigen (EMA), glucose transporter protein 1 (GLUT-1), insulin-like growth factor 2 messenger RNA-binding protein 3 (IMP-3), and CD146, have been proposed to assist in uncertain cases (17-24). Nevertheless, none of these markers, alone or in combination, appeared to be useful with sufficient confidence in the routine diagnosis of MPM (6).
Among new ancillary tests, FISH that shows the homozygous deletion of p16 (CDKN2A) and the loss of BRCA1-associated protein 1 (BAP1) expression by immunocytochemistry are particularly useful to differentiate mesothelial hyperplasia (MH) from MPM. These two markers were shown to be highly specific for MPM; however, their low sensitivity limits their clinical utility (25-32).
The cytological distinction between mesothelioma and secondary carcinoma is less problematic now than in earlier decades; overall, the sample is adequate for cell block preparation for various immunohistochemical studies (33). Because of the frequent litigation of cases of MPM and the availability of many mesothelial and adenocarcinoma markers, the guidelines strongly recommend that all cases should be confirmed with ICC/IHC (7).
Histologic patterns of MPM
MPM is a heterogeneous tumor, including three main histological subtypes: epithelioid (60–80%), sarcomatoid (<10%), biphasic/mixed (10–15%), and other less common ones (33). The recognition of the various histopathologic patterns facilitates differential diagnosis and subsequent ancillary tests. The invasion of the chest wall soft tissue or underlying lung parenchyma is still the most reliable indicator of malignancy.
MPM typically presents with lung encasement and relative sparing of the lung parenchyma, but pathologists should be aware of unusual presentations, including MPM cases with absent or scarce pleural involvement and presentation as metastatic disease or mimicking interstitial lung diseases (34).
Epithelioid MPM shows a wide range of histological patterns, and several distinct patterns are observed in the same neoplasm, although one pattern may predominate. Recently, the clinical and prognostic significance of many of these patterns has been demonstrated (35-37). Moreover, Kadota and collaborators proposed a nuclear grading system for epithelioid MPM with a prognostic value independent from histologic patterns (38). However, further studies are necessary to clinically validate this nuclear grading system.
Commonly, epithelioid tumors contain polygonal, oval or cuboidal cells that often mimic reactive mesothelial cells that occur in response to various types of injury. Mitoses are infrequent except for the more poorly differentiated epithelioid neoplasms, which are uncommon (6).
The most common secondary histological patterns of epithelioid MPM are tubulopapillary, solid, and trabecular, and psammoma bodies may present in any of these patterns (Figure 2A). Other less common patterns include micropapillary, adenomatoid (microcystic), clear cells, transitional, deciduoid, small cells, and lymphohistiocytic (33,39). Recently, a pleomorphic variant of MPM was identified, in which the cells show marked nuclear pleomorphisms in more than 10% of the tumors. Two different studies have shown that this pattern has highly aggressive behavior and poor survival, like that of sarcomatoid MPM (35,40). Additionally, the high-grade subgroup with a deciduoid pattern shows a much more aggressive clinical course (41). The fibrous reactive stroma present in epithelioid MPM can be scant or prominent, with various grades of cellularity that could make the distinction from true sarcomatoid component difficult. In these cases, BAP-1 IHC can be helpful, showing a loss of expression in areas of sarcomatoid mesothelioma (31).
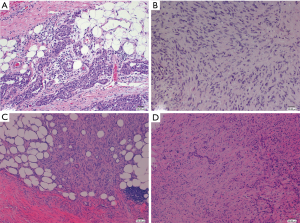
Sarcomatoid mesothelioma is the least common but most aggressive of the three histological types of mesothelioma (42). The sarcomatoid pattern of MPM is usually characterized by the proliferation of spindle cells arranged in fascicles or haphazardly, with various grades of nuclear atypia and mitotic activity. The sarcomatoid tissue rarely shows heterologous differentiation, such as osteoid, bone or cartilage (33,43) (Figure 2B).
Desmoplastic MPM is characterized by proliferation in at least 50% of the bland spindle cells arranged in a “patternless” pattern within a band of dense collagenous stroma. The histological distinction between desmoplastic MPM and benign fibrous pleuritis can be difficult. In addition to invasion, histological criteria that indicate malignancy include cellular stromal nodules and bland necrosis or areas of epithelioid and sarcomatoid MPM (33,44) (Figure 2C).
Biphasic malignant mesotheliomas contain a mixture of epithelioid and sarcomatoid areas within the same tumor. Each pattern should constitute at least 10% of the neoplasm; when there is less of either, the malignant mesothelioma can be designated predominantly sarcomatoid or epithelioid (33) (Figure 2D).
Immunohistochemical markers for the diagnosis of MPM
IHC is integral to the diagnosis of MPM, representing the most useful and standard ancillary procedure to distinguish this malignancy from other types of cancer. The exact combination and number of antigens to evaluate is dependent on the histopathological patterns of MPM (epithelioid/sarcomatoid), the diagnostic dilemma to be resolved, and on the antibodies available in the pathology laboratory (45,46). Since none of the antibodies used for the diagnosis of MPM is 100% sensitive or specific, the International Mesothelioma Interest Group (IMIG) recommends an initial workup with an immunohistochemical panel comprising pancytokeratin (multiple keratins, such as AE1/AE3, CAM5.2) plus two mesothelial markers and two markers for the other tumor under consideration based on morphology. If the results are concordant, the diagnosis could be considered conclusive. If the results of this immunohistochemical panel are discordant, the pathologist should expand the panel of antibodies, always based on the differential diagnosis to resolve (6). The immunohistochemical markers should have sensitivity or specificity greater than 80%, and the interpretation of immunostains should consider the localization of the stain (membrane, nuclear, cytoplasmic) and the percentage of positive cells, in which more than 10% has been suggested for cytoplasmic membranous markers (6).
Immunohistochemical staining with pancytokeratin is particularly useful in the diagnosis of MPM, since all mesotheliomas potentially show positive results. However, few (approximately 5–10%) sarcomatoid mesotheliomas are keratin-negative; in these cases, other mesothelial markers, such as calretinin and podoplanin (D2-40), could lead to the exact diagnosis (36,42). Based on their sensitivity and specificity, the most useful mesothelial markers to support a MPM diagnosis are calretinin, Wilms’ tumor gene (WT1), cytokeratin 5/6 (CK5/6), and D2-40 (6,33). However, negativity for the mentioned mesothelial antibodies does not exclude the diagnosis of MPM, since 30% of MPM presents a “null” phenotype (3). The choice of the other immunohistochemical markers included in the diagnostic panel depends on the tumor in differential diagnosis (see next paragraphs for more details).
Differential diagnosis of benign and malignant mesothelial proliferations
Reactive MH versus epithelioid/mixed MPM
As emphasized earlier in this review, the definitive diagnosis of MPM requires stromal or lung invasion and mostly relies on histological examination, with the exception of some cytological specimens. The differential diagnosis of benign from malignant mesothelial proliferations is crucial to patient care and has medical-legal implications because of the occupational relationship between MPM and asbestos exposure (14,46,47). Although reactive mesothelial proliferations are non-invasive, the entrapment of benign mesothelial cells within fibrous tissue can simulate neoplastic invasion. Therefore, this differential diagnosis is often morphologically difficult, making it necessary to resort to various ancillary tests.
As mentioned above, several immunohistochemical markers are more likely to be positive in benign proliferations and others in malignant ones. These markers include desmin, p53, EMA, GLUT-1, IMP-3, and CD146 (17-24). However, there is insufficient evidence to rely upon in single cases (6).
Currently, BAP1 IHC and p16 FISH represent the most effective analyses to discriminate between benign and malignant pleural lesions (16,25-31).
BAP1 somatic mutations resulting in protein loss appear to be common in hereditary and sporadic mesotheliomas (25). Currently, there is considerable variability in the reported frequency of BAP1 protein loss; epithelioid/mixed mesotheliomas lose BAP1 more frequently than the sarcomatoid pattern, approximately 60–70% and 15%, respectively. Interestingly, recent studies have shown BAP1 protein expression in all benign mesothelial proliferations and, although more data are needed, the specificity of BAP1 loss is 100%, making BAP1 an excellent biomarker in the distinction between benign and malignant mesothelial proliferations (16,28-31).
Several recent studies have shown that the homozygous deletion of p16 by FISH is found only in mesotheliomas, whereas none of benign mesothelial proliferations showed a loss of p16 with a specificity of 100% (16,25-27). However, not all mesotheliomas harbor this deletion, and the sensitivity for epithelioid/biphasic MPM ranges from approximately 45% to 85%. The sensitivity of the p16 FISH test is much higher in sarcomatoid mesothelioma; in some reports, the deletion is reported in up to 100% of cases; however, other studies report a lower proportion of p16-deleted sarcomatous tumors (16).
Beyond the excellent specificity of these two markers, their low sensitivity limits their clinical utility, as the failure to identify p16 loss by FISH or BAP1 loss by IHC does not make a process benign. Recently, it has been reported that the limited sensitivity of each test may be improved by running both of them (32,48,49).
Fibrous organizing pleuritis versus desmoplastic MPM
Desmoplastic mesotheliomas are paucicellular processes that look like scars or organizing pleuritis at low power. The separation of benign fibrous entities from desmoplastic MPM could be extremely difficult (6,33,44,47). The invasion into adjacent tissue by neoplastic cells is often more difficult to visualize than in other histological types of MPM. Immunostaining for pancytokeratin is helpful to highlight the presence of the malignant cells into the stromal tissue. However, the pathologist should be careful to not confuse the true invasion of desmoplastic MPM with the fatlike spaces that may be present in some organizing pleuritis (50). This change is the result of traction artifact caused by inflammation and organization in the fibrous connective tissue. S-100 IHC can help to distinguish true fat from fake fat, which are usually positive and negative, respectively (50).
In addition to stromal invasion, histological features that could be useful in this differential diagnosis include the uniformity of growth in organizing pleuritis with typical zonation constituted by increased cellular infiltrate under the effusion and less cellular infiltrate with more fibrosis towards the chest wall. Another feature is the presence of pleuritis with small capillaries oriented perpendicular to the surface opposite to the inconspicuous capillaries in the tumor. Furthermore, desmoplastic MPM could show nodular stromal expansions, foci of frank sarcomatoid or epithelioid MPM, and bland tumor necrosis (14). Interestingly, molecular analysis of p16 by FISH could be particularly advantageous in the differential diagnosis of desmoplastic mesothelioma due to the high frequency of p16 homozygous deletion reported in the literature in this variant of MPM. On the other hand, immunohistochemical BAP1 loss is rarely present in sarcomatous and desmoplastic mesothelioma, demonstrating its limited value in this setting (51).
Differential diagnosis of MPM and secondary tumors involving the pleura
Epithelioid/mixed malignant mesothelioma versus carcinoma
The differential diagnosis between epithelioid/mixed malignant mesothelioma and metastatic carcinoma to the pleura varies in relation to the morphological and clinical information, which guide the selection of the IHC panel. Indeed, IHC can greatly improve this diagnostic topic (6,33,45).
The most useful general carcinoma markers are carcinoembryonic antigen (CEA), BerEP4, CD15 (LeuM1), MOC31, BG8, claudin 4, and B72.3 (24,33,45,46). Other immunohistochemical markers can be used to confirm the origin of carcinoma. The primary differential diagnosis for epithelioid MPM is metastatic lung adenocarcinoma; in this case, the immunohistochemical panel should include the markers of lung adenocarcinoma thyroid transcription factor 1 (TTF-1) and napsin A (52). p40 is the best marker to distinguish MPM from squamous cell carcinoma, whereas CK5/6 does not solve this diagnostic dilemma since it is also expressed in MPM (53,54). Estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, if positive markers, can be helpful in distinguishing MPM from metastatic breast carcinoma (55). PAX8 or PAX2 nuclear positivity differentiates renal cell carcinoma from MPM since they are not expressed in the mesothelial neoplasm (56). Adenocarcinoma of the gastrointestinal tract and of the prostate can be differentiated by CDX2 nuclear positivity and prostate specific antigen (PSA) cytoplasmic staining, respectively (46).
Sarcomatoid malignant mesothelioma versus spindle cell malignancy
The major differential diagnoses for sarcomatoid MPM are primary and secondary sarcoma and metastatic sarcomatoid carcinoma (39).
IHC has a more restricted role for the differential diagnosis of sarcomatoid MPM than for the epithelioid/mixed form, since mesothelial markers often show weak and focal expression or fail to identify mesothelial differentiation. The most useful markers for sarcomatoid MPM are calretinin and D2-40, which are expressed in a variable percentage of cases and which can recognize the mesothelial origin of the neoplasm (3,45). Most sarcomatoid/desmoplastic MPMs are strongly positive for cytokeratins, whereas most sarcomas are keratin-negative. Thus, consistent keratin immunostaining combined with calretinin and D2-40 could be useful to distinguish spindle cell mesothelioma from sarcoma of a different lineage (57-59). Occasionally, the expression of muscle markers (muscle-specific actin, smooth muscle actin, desmin) and/or neural markers (S-100, neuron-specific enolase) can be observed in sarcomatoid MPM. Therefore, the demonstration of positive staining for keratin and mesothelial markers is essential to confirm the diagnosis of MPM (43). Moreover, there are some keratin-positive sarcomas, such as angiosarcoma and monophasic synovial sarcoma. In these cases, the expression of specific lineage markers and the presence of characteristic genetic changes could solve diagnostic issues (57-59). In particular, monophasic synovial sarcoma is keratin- and calretinin-positive; then, the identification of the chromosomal translocation t(X;18) is of great aid when this entity enters differential diagnosis (60).
Clearly, positive results for keratins alone do not rule out a metastatic sarcomatoid carcinoma. In this setting, the positivity of mesothelial markers (calretinin, D2-40) supports the diagnosis of sarcomatoid MPM (45).
Discussion
The diagnostic process of MPM is complex and can be one of the greatest challenges faced by the practicing surgical pathologist. The definitive pathological diagnosis of MPM usually requires a tissue specimen (and, less frequently, cytology) to demonstrate that the tumor has a mesothelial phenotype and that it shows neoplastic invasion as opposed to reactive MH. Evidence of MPM on cytological examination should be confirmed with histological analysis, or if biopsy is not feasible, the cytological diagnosis should be always supported by clinical, radiologic, and surgical findings.
The identification of the histological appearance (epithelioid, biphasic, sarcomatoid) of MPM could facilitate diagnosis and provide important prognostic information since the histotype is still the best predictor of prognosis.
IHC is fundamental for the diagnosis and differential diagnosis of MPM. The immunohistochemical approach should rely on the application of a panel including positive (mesothelial-related) and negative markers as suggested by morphology and clinical information when available. Moreover, molecular analysis, such as a FISH assay for the p16 homozygous deletion, and immunohistochemical evaluation of BAP1 expression could be useful in selected cases, distinguishing benign from malignant pleural proliferations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 2013;34:1413-9. [Crossref] [PubMed]
- Novello S, Pinto C, Torri V, et al. The Third Italian consensus conference for malignant pleural mesothelioma: state of the art and recommendations. Crit Rev Oncol Hematol 2016;104:9-20. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian consensus conference on malignant pleural mesothelioma: state of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Hjerpe A, Ascoli V, Bedrossian C, et al. Guidelines for cytopathologic diagnosis of epithelioid and mixed type malignant mesothelioma. complementary statement from the international mesothelioma interest group, also endorsed by the international academy of cytology and the papanicolaou society of cytopathology. Cytojournal 2015;12:26. [Crossref] [PubMed]
- Rakha EA, Patil S, Abdulla K, et al. The sensitivity of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Diagn Cytopathol 2010;38:874-9. [Crossref] [PubMed]
- Paintal A, Raparia K, Zakowski MF, et al. The diagnosis of malignant mesothelioma in effusion cytology: a reappraisal and results of a multi-institution survey. Cancer Cytopathol 2013;121:703-7. [Crossref] [PubMed]
- Segal A, Sterrett GF, Frost FA, et al. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20 year audit. Pathology 2013;45:44-8. [Crossref] [PubMed]
- Henderson DW, Reid G, Kao SC, et al. Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol 2013;66:847-53. [Crossref] [PubMed]
- Ismail-Khan R, Robinson LA, Williams CC Jr, et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control 2006;13:255-63. [Crossref] [PubMed]
- Galateau-Salle F, Churg A, Roggli V, et al. World Health Organization Committee for Tumors of the Pleura. The 2015 World Health Organization classification of tumors of the pleura: advances since the 2004 classification. J Thorac Oncol 2016;11:142-54. [Crossref] [PubMed]
- Churg A, Galateau-Salle F. The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med 2012;136:1217-26. [Crossref] [PubMed]
- Husain AN. Mesothelial proliferations: useful marker is not the same as a diagnostic one. Am J Clin Pathol 2014;141:152-3. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New markers for separating benign from malignant mesothelial proliferations: are we there yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 2003;43:231-8. [Crossref] [PubMed]
- Minato H, Kurose N, Fukushima M, et al. Comparative immunohistochemical analysis of IMP3, GLUT1, EMA, CD146, and desmin for distinguishing malignant mesothelioma from reactive mesothelial cells. Am J Clin Pathol 2014;141:85-93. [Crossref] [PubMed]
- Shi M, Fraire AE, Chu P, et al. Oncofetal protein IMP3, a new diagnostic biomarker to distinguish malignant mesothelioma from reactive mesothelial proliferation. Am J Surg Pathol 2011;35:878-82. [Crossref] [PubMed]
- Lee AF, Gown AM, Churg A. IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol 2013;37:421-6. [Crossref] [PubMed]
- Husain AN, Mirza MK, Gibbs A, et al. How useful is GLUT-1 in differentiating mesothelial hyperplasia and fibrosing pleuritis from epithelioid and sarcomatoid mesotheliomas? An international collaborative study. Lung Cancer 2014;83:324-8. [Crossref] [PubMed]
- Ikeda K, Tate G, Suzuki T, et al. Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn Cytopathol 2011;39:395-401. [Crossref] [PubMed]
- Ikeda K, Tate G, Suzuki T, et al. IMP3/L523S, a novel immunocytochemical marker that distinguishes benign and malignant cells: the expression profiles of IMP3/L523S in effusion cytology. Hum Pathol 2010;41:745-50. [Crossref] [PubMed]
- Lonardi S, Manera C, Marucci R, et al. Usefulness of claudin 4 in the cytological diagnosis of serosal effusions. Diagn Cytopathol 2011;39:313-7. [Crossref] [PubMed]
- Ladanyi M. Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer 2005;49:S95-8. [Crossref] [PubMed]
- Illei PB, Ladanyi M, Rusch VW, et al. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 2003;99:51-6. [Crossref] [PubMed]
- Chung CT, Santos Gda C, Hwang DM, et al. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J Clin Pathol 2010;63:630-4. [Crossref] [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868-74. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumours of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Larsen BT, Klein JR, Hornychová H, et al. Diffuse intrapulmonary malignant mesothelioma masquerading as interstitial lung disease: a distinctive variant of mesothelioma. Am J Surg Pathol 2013;37:1555-64. [Crossref] [PubMed]
- Kadota K, Suzuki K, Sima CS, et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896-904. [Crossref] [PubMed]
- Arif Q, Husain AN. Malignant Mesothelioma Diagnosis. Arch Pathol Lab Med 2015;139:978-80. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of epithelioid differentiation is a predictor of survival in malignant pleural mesothelioma. Ann Thorac Surg 2017;103:962-6. [Crossref] [PubMed]
- Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71. [Crossref] [PubMed]
- Churg A, Cagle PT, Roggli VL. Tumors of the Serosal Membranes. Armed Forces Institute of Pathology Atlas of Tumor Pathology 2006;4th series, fascicle 3. Occup Environ Med 2007;64:288.
- Ordóñez NG. Pleomorphic mesothelioma: report of 10 cases. Mod Pathol 2012;25:1011-22. [Crossref] [PubMed]
- Ordóñez NG. Deciduoid mesothelioma: report of 21 cases with review of the literature. Mod Pathol 2012;25:1481-95. [Crossref] [PubMed]
- Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9. [Crossref] [PubMed]
- Klebe S, Mahar A, Henderson DW, et al. Malignant mesothelioma with heterologous elements: clinicopathological correlation of 27 cases and literature review. Mod Pathol 2008;21:1084-94. [Crossref] [PubMed]
- Mangano WE, Cagle PT, Churg A, et al. The diagnosis of desmoplastic malignant mesothelioma and its distinction from fibrous pleurisy: a histologic and immunohistochemical analysis of 31 cases including p53 immunostaining. Am J Clin Pathol 1998;110:191-9. [Crossref] [PubMed]
- Betta PG, Magnani C, Bensi T, et al. Immunohistochemistry and molecular diagnostics of pleural malignant mesothelioma. Arch Pathol Lab Med 2012;136:253-61. [Crossref] [PubMed]
- Ordóñez NG. Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol 2013;44:1-19. [Crossref] [PubMed]
- Churg A, Colby TV, Cagle P, et al. The separation of benign and malignant mesothelial proliferations. Am J Surg Pathol 2000;24:1183-200. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol 2016;40:714-8. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. BAP1 immunohistochemistry and p16 FISH results in combination provide higher confidence in malignant pleural mesothelioma diagnosis: ROC analysis of the two tests. Pathol Int 2016;66:563-70. [Crossref] [PubMed]
- Churg A, Cagle P, Colby TV, et al. US-Canadian Mesothelioma Reference Panel. The fake fat phenomenon in organizing pleuritis: a source of confusion with desmoplastic malignant mesotheliomas. Am J Surg Pathol 2011;35:1823-9. [Crossref] [PubMed]
- Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the diagnosis of malignant mesothelioma in effusion cytology specimens. Am J Surg Pathol 2016;40:120-6. [Crossref] [PubMed]
- Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum Pathol 2010;41:20-5. [Crossref] [PubMed]
- Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol 2006;19:417-28. [Crossref] [PubMed]
- Tatsumori T, Tsuta K, Masai K, et al. p40 is the best marker for diagnosing pulmonary squamous cell carcinoma: comparison with p63, cytokeratin 5/6, desmocollin-3, and sox2. Appl Immunohistochem Mol Morphol 2014;22:377-82. [Crossref] [PubMed]
- Ordóñez NG, Sahin AA. Diagnostic utility of immunohistochemistry in distinguishing between epithelioid pleural mesotheliomas and breast carcinomas: a comparative study. Hum Pathol 2014;45:1529-40. [Crossref] [PubMed]
- Ordóñez NG. Value of PAX8, PAX2, napsin A, carbonic anhydrase IX, and claudin-4 immunostaining in distinguishing pleural epithelioid mesothelioma from metastatic renal cell carcinoma. Mod Pathol 2013;26:1132-43. [Crossref] [PubMed]
- Lucas DR, Pass HI, Madan SK, et al. Sarcomatoid mesothelioma and its histological mimics: a comparative immunohistochemical study. Histopathology 2003;42:270-9. [Crossref] [PubMed]
- Rdzanek M, Fresco R, Pass HI, et al. Spindle cell tumors of the pleura: differential diagnosis. Semin Diagn Pathol 2006;23:44-55. [Crossref] [PubMed]
- Beasley MB. Immunohistochemistry of pulmonary and pleural neoplasia. Arch Pathol Lab Med 2008;132:1062-72. [PubMed]
- Miettinen M, Limon J, Niezabitowski A, et al. Calretinin and other mesothelioma markers in synovial sarcoma: analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol 2001;25:610-7. [Crossref] [PubMed]


