Outcomes of perioperative extracorporeal membrane oxygenation use in patients undergoing lung transplantation
Introduction
Following pivotal progress in technical improvements and safety as demonstrated in the CESAR trial (1) and other recent encouraging survival data (2), extracorporeal membrane oxygenation (ECMO) is now considered in a broader spectrum of adult patients with respiratory failure, and the feasibility and advantages of its use as a bridge to lung transplantation (LTx) have been suggested by an increasing number of encouraging findings (3). Moreover, there has been a notable paradigm shift from intraoperative cardiopulmonary bypass (CPB) to ECMO for intraoperative cardiopulmonary support during LTx. In line with several theoretical advantages of ECMO compared with CPB, including a miniature circuit requiring a closed circulation circuit with no cardiotomy suction and enhanced biocompatibility leading to lesser anticoagulation requirement that potentially lowers the activation of the coagulation cascade and inflammatory system (4), increasing data on the comparison of intraoperative ECMO with CPB favors the use of ECMO (5-9). We have recently reported our experience of the transition to routine use of intraoperative venoarterial ECMO (vaECMO) from CPB with encouraging results. The purpose of this single-center study was to review the transplant outcomes of patients receiving LTx using intraoperative ECMO according to the perioperative use of ECMO.
Methods
Patients
Intraoperative cardiopulmonary support using vaECMO has been used routinely for all patients undergoing LTx at our institution since March 2013 (10). We conducted a retrospective review of clinical data on 107 consecutive adult patients (>18 years of age) who underwent LTx with intraoperative vaECMO between March 2013 and August 2016 at Yonsei University Severance Hospital (Seoul, Korea). Pre-, intra-, and post-operative data and laboratory and respiratory data were obtained at the initiation and during ECMO therapy using a retrospective review of the prospectively recorded registry database and electronic medical records. This study was approved by the Institutional Review Board of Yonsei University Health Service at Severance Hospital and complied with the Declaration of Helsinki.
Definition
Extended ECMO use was defined if the patient left the operative room with a running ECMO system, and secondary ECMO use was defined if the patient required re-insertion of ECMO after decannulation (5). Bridging ECMO use was defined if the patient received a bridging therapy with ECMO before LTx. Primary graft dysfunction (PGD) was assessed and graded according to the International Society for Heart and LTx lung transplant injury grades (11), after excluding other potential diagnoses as previously reported (12). We considered PGD recorded between 48 and 72 hours after LTx for the analysis.
LTx and ECMO technique
The operative technique and general principle for LTx have previously been described (10,12). Briefly, the surgical approaches were either bilateral anterolateral thoracotomy preferably in the fourth intercostal space for single- (n=8) and double- (n=99) LTx. All patients received standard triple immunosuppressant consisting of calcineurin inhibitor (cyclosporine or tacrolimus), mycophenolate mofetil, and methylprednisolone. All intraoperative cardiopulmonary support was performed via vaECMO, while those who were initially on bridging veno-veno ECMO (vvECMO) required a change to vvaECMO (veno-veno-arterial ECMO). vaECMO was established during anesthesia induction or after open-chest or hilar dissection. Following a heparin infusion of 2,000 units, patients were cannulated peripherally, centrally, or a combination of both. The target activated clotting time was 150–180 seconds during ECMO. Protamine was used only on weaning from intraoperative ECMO. Other institutional procedures regarding ECMO techniques were performed as described previously (10).
Statistical analysis
Continuous variables were compared using the Kruskal-Wallis test and shown as the median with interquartile range (IQR). Categorical data were analyzed using Fisher’s exact test or Pearson’s χ2 test, depending on the distribution, and these data are shown as absolute frequencies and percentages. The overall survival (OS) curve after LTx was plotted using the Kaplan-Meier method, and differences were compared using the log-rank test. All statistical analyses were performed using Statistical Package for the Social Sciences version 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient demographics
During the study period, a total of 107 adult patients (median age 55 years, range, 18–75 years; male sex, n=64) were transplanted using ECMO for intraoperative cardiopulmonary support. Indications for LTx were idiopathic pulmonary fibrosis (IPF; n=62), connective tissue disease ILD (n=9), COPD (n=6), bronchiectasis (n=5), bronchiolitis obliterans syndrome after allogeneic stem cell transplantation (n=12), acute interstitial pneumonitis (n=4), pulmonary hypertension (n=3), lymphangioleiomyomatosis (n=3) and other indications (n=3).
Perioperative use of ECMO support
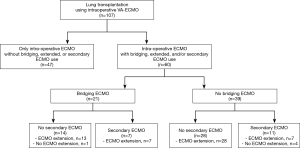
The patient population was grouped as summarized in Figure 1. Of 107 patients undergoing LTx using an intraoperative ECMO, 60 (56.1%) patients received additional ECMO support. That is, 21 of these 60 patients (35%) were on ECMO for a bridging therapy before transplant and 18 patients (30.0%) required a secondary use of ECMO support after decannulation; whereas, in 55 of the patients (91.7%), ECMO support was extended into the postoperative period with a configuration change from vvaECMO to vvECMO. The remaining 47 patients received ECMO support only intraoperatively (43.9%) and were weaned off from ECMO in operation room.
Survival according to the perioperative use of ECMO support
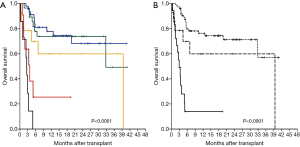
We first analyzed the OS according to the perioperative uses of ECMO, arbitrary divided into five clinical situations: (I) 47 (43.9%) patients receiving only intraoperative ECMO use; (II) 28 (26.2%) patients with extended use of ECMO but without bridging ECMO nor second ECMO; (III) 14 (13.1%) patients receiving bridging ECMO with (n=13) or without (n=1) extended ECMO but not requiring a second ECMO; (IV) 11 (10.3%) patients receiving a second ECMO with (n=7) or without (n=4) extended ECMO but no bridging ECMO; (V) 7 (6.5%) patients with bridging and secondary ECMO (all required extended use). After observing the OS of each group [1 year OS, group (I): 77.8%±6.6%; group (II): 73.8%±8.5%; (III): 60.0%±14.3%; (IV): 25.5%±15.1%; (V): 0%] as shown in Figure 2A, we merged the patients into three arbitrary groups: Group A (n=75, 70.1%), patients receiving only intraoperative ECMO or extended ECMO but no bridging nor second ECMO [(I) + (II)]; Group B (n=14, 13.1%), patients receiving bridging ECMO but no second ECMO (III); and Group C (n=18, 16.8%), patients receiving a second ECMO with (n=7) or without (n=11) a bridging ECMO [(IV) + (V)].
Comparisons of clinical characteristics and transplant outcomes in Group A, B, and C
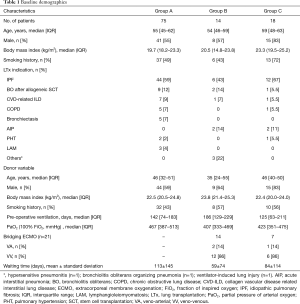
Baseline demographics of patients and donors were similar among the three groups, as summarized in Table 1. Patients who required re-implantation of secondary ECMO after LTx (Group C) showed male predominance and higher body mass index, while patients in Group A, who did not receive bridging ECMO nor secondary ECMO, had donors with higher PaO2 values compared to Groups B and C. The waiting time was longer in Group A, as compared with Groups B and C (mean, 113 vs. 59 vs. 84 days; Table 1).
Full table
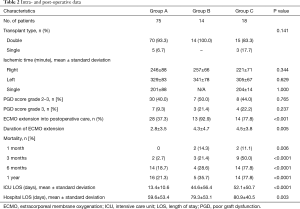
PGD score grade 3 (n=14), which was assessed at the time period between 48 and 72 hours after transplant, was more frequently observed in Group B (21.4%) and in Group C (22.2%) when compared with that in Group A (9.3%), without a statistical significance (Table 2). On the other hand, 13 of 14 patients (92.9%) with PGD score grade 3 had intraoperative ECMO extension, while 45.2% of the remaining non-PGD-grade 3 patients (42/93) required ECMO extension (P=0.001; data not shown). With regard to early post-LTx outcomes, when compared with Groups B or C, patients in Group A had a significantly shorter duration of intraoperative ECMO extension (mean, 2.8 vs. 4.3 vs. 4.5 days; P=0.005), shorter intensive care unit (ICU) length of stay (LOS; mean, 13.4 vs. 44.6 vs. 52.1 days; P<0.0001), and shorter hospital LOS (mean, 59.6 vs. 79.3 vs. 80.9; P=0.003).
After a median of 17.7 months (range, 3.1–40.9 months) for survivors, the one year OS rates after LTx according to the three groups were 76.3%±5.2% for Group A, 59.9%±14.3% for Group B, and 14.0%±9.0% for Group C (P<0.0001; Figure 2B). Mortality rates at 1, 3, and 6 months after LTx were significantly higher in Group C (Table 2). The characteristics of each patient in Group C who received re-implantation of secondary ECMO are summarized in Table 3. The most common cause of second ECMO was acute respiratory failure (n=12; 67%), and the mean number of days from LTx to implantation of the second ECMO was 7.9±5.3 days. Successful weaning from the second ECMO was observed in nine patients; however, five of these patients did not survive (Table 3). The time from LTx to secondary ECMO was longer for the patients with weaning failure when compared with those who were successfully weaned (57.0±44.8 vs. 30.0±35.8; P=0.101). Causes of mortality are summarized in Table S1. In our cohort, infection was the most common cause of death: Group A, n=12 of 19 deaths (63%); Group B, n=3 of 6 deaths (50%); and Group C, n=11 of 14 deaths (79%), and the most common pathogen were Acinetobacter baumannii (n=11), followed by Pseudomonas aeruginosa (n=6) and Klebsiella pneumonia (n=5; Table S1). The ICU LOS and hospital LOS were significantly longer for the 27 patients with infection-related deaths, when compared with that of the other patients (n=81; mean for ICU LOS, 52.6 vs. 14.8 days, P<0.0001; mean for hospital LOS, 96.5 vs. 55.9 days, P<0.0001).
Full table
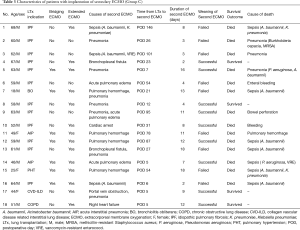
Full table
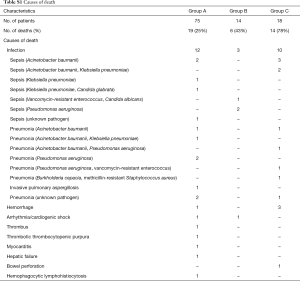
Full table
Clinical characteristics and transplant outcomes of patients with pretransplant bridging ECMO
Regarding 21 patients with preoperative bridging ECMO (all 14 patients in Group B and 7 patients of Group C), the type of ECMO was VA-ECMO in 2 patients and VV ECMO in the remaining 19 patients. The mean duration of preoperative ECMO bridging was 16.4±15.6 (IQR, 5.5–19.5), whereas the mean waiting time was significantly shorter for patients with bridging ECMO (63.2±95.4 days; IQR 7.0–78.5), compared with those without bridging ECMO (110.1±140.3 days; IQR 23.3–142.3; P=0.022). The most common indication of LTx in the 21 patients with bridging ECMO was IPF (n=9, 42.9%), followed by AIP (n=4, 19.0%). ECMO support was extended postoperatively in all but one patient with bridging ECMO, whereas PGD grade 3 was observed more frequently in patients with bridging ECMO when compared with the others (28.6% vs. 9.3%; P=0.030). The mean ICU LOS and hospital LOS for the pretransplant ECMO group were significantly longer than for the other patients (mean for ICU LOS, 57.7±56.1 vs. 15.7±18.6 days, P<0.0001; mean for hospital LOS, 86.6±48.7 vs. 60.7±51.6 days, P=0.002). While the OS at 1 year was 38.9%±11.4% for all 21 patients with pretransplant ECMO, there was a significant difference in OS for patients without requiring (Group B, n=14; 60.0%±14.3%) versus those with (7 of 18 patients in Group C; all died within a year after LTx) secondary ECMO support (P=0.003; Figure 2A).
Discussion
Recently, increasing experience of ECMO has been gained in terms of pre-, intra-, and postoperative cardiopulmonary support for patients undergoing LTx, with favorable results. Regarding intraoperative ECMO during LTx, Ius et al. demonstrated lower blood product transfusion requirements and improved survival in the ECMO group, when compared with the CPB group (8); whereas Aigner et al. suggested that the versatility of ECMO allows maintaining ECMO utilized intraoperatively can be extended in the postoperative period (5). However, data regarding the optimal approach of ECMO support in the postoperative period of LTx remain limited, heterogeneous in the setting of its use, and with varying success in results (13-16). Following the routine use of intraoperative VA-ECMO in our institution (10), this present study examined our single-center experience of transplant outcomes in 107 patients undergoing an LTx with the use of ECMO support in the perioperative period as a bridge to transplant, as an intraoperative cardiopulmonary support, and/or as a postoperative bridge to recovery.
Postoperative extended ECMO support was not used electively in any of our patients, and one can expect that the patients who did not require additional ECMO support other than ECMO as intraoperative cardiopulmonary support would show the best clinical outcomes. By separately considering those with pretransplant ECMO support or posttransplant secondary ECMO implantation, we demonstrated that the patients with extended use of the intraoperative ECMO had similar (P=0.856) survival rates compared with the patients with intraoperative ECMO only, with respective OS rates at 6-month and 1-year of 81% vs. 78% and 78% vs. 74%, respectively. Previous studies reporting outcomes of post-LTx ECMO support have typically dealt with ECMO as a rescue strategy that is mainly utilized for early severe PGD. Moreover, before intraoperative ECMO was widely accepted, post-LTx ECMO support meant that the implantation of ECMO devices was required after LTx and that the best time to start post-LTx ECMO had to be determined. According to the study by Hartwig et al., 28 of 498 (6%) study patients undergoing LTx required VV-ECMO implantation for severe PGD after transplant, and 96% of these patients were successfully weaned with one year OS of 64% (14). Initiation of postoperative ECMO was considered when mechanical ventilator requirements reached peak inspiratory pressures of 35 cm H2O and the fraction of inspired oxygen content surpassed 0.60 in their study (14). In contrast, Bermudez et al. reported that 67% (n=39) of 58 patients who required early (0 to 7 days after lung or heart-lung transplant) ECMO support after LTx for PGD were successfully weaned with one year OS of 59%. In their cohort, ECMO was usually initiated when PaO2 was <60 mmHg with a FiO2 >80% (13). While there is a possibility that earlier institution of post-LTx ECMO support could favorably affect LTx not using intraoperative ECMO, recent reports showed that the prevalence of PGD and the need for secondary ECMO implantation could be reduced by continuing intraoperative ECMO support postoperatively (8,17). In our study, 53 of 55 patients (96%) were successfully weaned from the extended use of intraoperative ECMO support, although 14 of these patients (26%) eventually required a secondary ECMO after a mean of 32±39 days (IQR 3–55; data not shown).
On the other hand, the clinical outcomes of secondary use of postoperative ECMO support were significantly worse than the other subgroups of patients in our study. The main indication of secondary ECMO implantation after LTx in our cohort was pneumonia or sepsis, which is in agreement with a recent study by Marasco et al. (16). In their cohort, the indications for early ECMO (<7 days posttransplant) were primary graft failure, whereas the postoperative ECMO later than 7 days after LTx were established for infection and non-specific graft failure (16). Additionally, as suggested in the study by Harwig et al. in which blood stream infections appeared to be the main source of morbidity and mortality in patients with LTx, not using intraoperative ECMO, who required posttransplant ECMO support, the main causes of death was infection in our cohort (14). Mason et al. also identified that the only risk factor of mortality in their 22 patients who required post-LTx implantation of ECMO was the establishment of ECMO for sepsis or pneumonia (18). Moreover, multidrug-resistant organisms, including Pseudomonas aeruginosa, Acinetobacter baumannii, and extended-spectrum β-lactamase (ESBL)—producing or carbapenemase-producing Enterobacteriaceae (such as Klebsiella pneumoniae), were the main sources of sepsis or pneumonia in our study. It is not surprising that hospital-acquired infections in the ICU would be a significant obstacle for patients requiring a second ECMO implantation (19). Alternatively, Marasco et al. suggested that the innate disease processes determine the poor results seen for late secondary ECMO after LTx (16).
Waiting time before LTx is an important risk factor for transplant outcomes in patients awaiting LTx, particularly in cases at high risk of acute clinical decompensation that inevitably require prolonged mechanical ventilation prior to LTx (20,21). Owing to technical advancements (3) and promising results from the adoption of “awake ECMO” (22,23), pretransplant bridging ECMO for patients waiting for an available donor lung is now no longer an independent risk factor for mortality, although current recommendations regarding its indication are still based on institutional experience (3). Moreover, the implementation of the Lung Allocation Score led to improvement in waiting list mortality rates (24) and has given priority to patients undergoing pretransplant bridging ECMO because of their higher scores. In our cohort, ~20% of the patients were on ECMO preoperatively, suggesting the severity of these high-risk patients. In line with previous reports, our patients with bridging ECMO had significantly higher rates of PGD grade 3 (29% vs. 9%) and longer mean ICU (58 vs. 16 days) and hospital LOS (87 vs. 61 days). While the one year OS in all 21 patients with pretransplant bridging ECMO was relatively lower (~40%) than other recent reports (25), those who did not require a secondary ECMO showed an OS reaching 60%.
Limitations of this study include the retrospective nature of the analyses with a single center data and the unavailability of the data regarding parameters reflecting transplanted lung function. Moreover, decisions on the extension of intraoperative ECMO or the initiation of secondary ECMO were made on an individual basis, thus lacked predetermined criteria.
In conclusion, our data showed that the postoperative extended use of intraoperative ECMO during LTx is feasible and provides favorable survival outcomes similar to uncomplicated LTx with intraoperative ECMO only. However, poor survival outcomes shown for patients who required a secondary ECMO indicate that development of a solid strategy to reduce the need for secondary ECMO implantation after LTx seems important.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB) of Severance Hospital (No. 4-2013-0770).
References
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Brogan TV, Thiagarajan RR, Rycus PT, et al. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 2009;35:2105-14. [Crossref] [PubMed]
- Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis 2014;6:1070-9. [PubMed]
- Machuca TN, Cypel M, Keshavjee S. Cardiopulmonary Bypass and Extracorporeal Life Support for Emergent Intraoperative Thoracic Situations. Thorac Surg Clin 2015;25:325-34. [Crossref] [PubMed]
- Aigner C, Wisser W, Taghavi S, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg 2007;31:468-73; discussion 473-4. [Crossref] [PubMed]
- Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg 2014;98:1936-42; discussion 1942-3.
- Biscotti M, Yang J, Sonett J, et al. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2014;148:2410-5. [Crossref] [PubMed]
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Yu WS, Paik HC, Haam SJ, et al. Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes. J Thorac Dis 2016;8:1712-20. [Crossref] [PubMed]
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454-9. [Crossref] [PubMed]
- Moon S, Park MS, Lee JG, et al. Risk factors and outcome of primary graft dysfunction after lung transplantation in Korea. J Thorac Dis 2016;8:3275-82. [Crossref] [PubMed]
- Bermudez CA, Adusumilli PS, McCurry KR, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg 2009;87:854-60. [Crossref] [PubMed]
- Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71. [Crossref] [PubMed]
- Wigfield CH, Lindsey JD, Steffens TG, et al. Early institution of extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation improves outcome. J Heart Lung Transplant 2007;26:331-8. [Crossref] [PubMed]
- Marasco SF, Vale M, Preovolos A, et al. Institution of extracorporeal membrane oxygenation late after lung transplantation - a futile exercise? Clin Transplant 2012;26:E71-7. [Crossref] [PubMed]
- Tudorache I, Sommer W, Kühn C, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015;99:451-8. [Crossref] [PubMed]
- Mason DP, Boffa DJ, Murthy SC, et al. Extended use of extracorporeal membrane oxygenation after lung transplantation. J Thorac Cardiovasc Surg 2006;132:954-60. [Crossref] [PubMed]
- Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010;362:1804-13. [Crossref] [PubMed]
- Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg 2011;141:1270-7. [Crossref] [PubMed]
- George TJ, Beaty CA, Kilic A, et al. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant 2012;31:1182-91. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Hook JL, Lederer DJ. Selecting lung transplant candidates: where do current guidelines fall short? Expert Rev Respir Med 2012;6:51-61. [Crossref] [PubMed]
- Chiumello D, Coppola S, Froio S, et al. Extracorporeal life support as bridge to lung transplantation: a systematic review. Crit Care 2015;19:19. [Crossref] [PubMed]


