Mitral valve prolapse: an underestimated cause of sudden cardiac death—a current review of the literature
Introduction
Sudden cardiac death (SCD) is the death from cardiovascular causes, heralded by abrupt loss of consciousness within one hour of the beginning of an acute change in cardiovascular status (1). The term is used when a potentially fatal heart condition (congenital or acquired) was known to be present during life or autopsy has revealed a cardiac or vascular disease as the potential cause of the event. Also, if no apparent extracardiac causes have been identified by post-mortem examination, and therefore an arrhythmic event is a probable cause of death (2).
Cardiovascular diseases are responsible for approximately 17 million deaths every year in the world, nearly 25% of which are SCD (2). Based on the current guidelines, the SCD rate is estimated to range from 1.40 per 100,000 person-years in women to 6.68 per 100,000 person-years in men (2). SCD in younger individuals has an estimated incidence of 0.46–3.7 events per 100,000 person-years corresponding to a rough estimate of 1,100–9,000 deaths in Europe and 800–6,200 deaths in the USA every year (2). Etiology of SCD includes ischemic heart disease, structural cardiac abnormalities, cardiomyopathies, and electrophysiological disorders. In certain situations, the origin is not clear. Therefore, the term ‘‘idiopathic ventricular fibrillation” is adopted (1).
Mitral valve prolapse (MVP) is characterized by a systolic displacement of one or both mitral leaflets below the mitral annulus plane into the left atrium (LA) (1). It was initially reported by Barlow in the 1960s as a phenomenon with auscultatory and cine-angiocardiographic findings, before the development of diagnostic echocardiography (1). There are several articles in the literature describing SCD in MVP patients, with a substantial percentage of asymptomatic young individuals (1,3-6).
This review aims to give a conceptual description of the association of MVP and SCD.
Materials and methods
The MEDLINE/PubMed database was searched for publications with the medical subject heading ‘‘mitral valve prolapse’’ and keywords ‘‘sudden’’ or ‘’arrhythmia’’ or “arrhythmic” or “arrest” or “arrhythmias” or “malignant”. Additional records were identified through scanning bibliographies of relevant articles. Our selection criteria were the English language, the cardiovascular relevance (publications irrelevant to MVP and SCD, were excluded), a time frame of the last 10 years [2007–2017], and the availability of full-text articles. We enrolled 33 articles. Our aiming was to review the correlation between MVP and SCD, the understanding of the pathophysiology, the risk stratification and the treatment strategy. A comprehensive PRISMA flow diagram with exclusion criteria is reported in Figure 1.
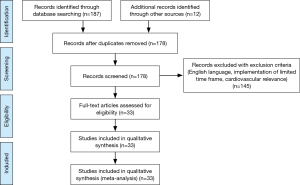
Results
Echocardiography
Echocardiography is useful for diagnosis, follow-up, and intervention evaluation of MVP. Carpentier’s functional classification of mitral regurgitation (MR) defined MVP (Type II excess leaflet motion) as an anomaly of leaflet motion, where one or different segments of the valve bulge into the LA during systole (1). Classic MVP is defined by >2 mm systolic displacement of one or both mitral valve leaflets into the LA in long-axis view, with a leaflet thickness of ≥5 mm. Non-classic MVP is defined by >2 mm leaflet displacement with a leaflet thickness of <5 mm (1,7).
Classic MVP has either a symmetric or an asymmetric site of coaptation. In symmetric MVP both leaflet tips are in the same position with the mitral valve annulus. Asymmetric coaptation results in one leaflet displacement towards the LA in comparison to the other leaflet. Asymmetric coaptation is more probable to worsen and cause flail prolapse, leading to increased severity of mitral insufficiency. Prolapse or flail segment illustrates the presence of leaflet tips that protrude into the LA. Flail prolapse may affect one leaflet, both leaflets (possibly secondary to chordal rupture), a single segment or multiple segments.
Both two-dimensional (2D) transthoracic (TTE) and transesophageal (TOE) echocardiography can be utilized to assess mitral valve apparatus (1,7). TOE provides a better view of the LA and should be under consideration in all situations of MVP evaluation. The diagnosis of MVP with TTE should only be established in the parasternal long-axis view, and the apical long-axis view as the paraboloid hyperbolic saddle-shaped surface of the mitral valve annulus can give a false-positive diagnosis (1). Additionally, a report of the leaflet thickness or redundancy, annular dilatation, and chordal length should be added. The visual accuracy of mitral valve apparatus and abnormality can be enhanced using three-dimensional (3D) echocardiography, particularly for the anterior leaflet or commissural involvement (1). Doppler imaging is vital to assess the severity of MR. The echocardiography study requires extensive methods to detect disease progression, predict outcome and evaluate appropriateness for intervention (1).
The subsequent effects of MR such as LV, LA, and RV dilatation, LV systolic dysfunction, pulmonary vein flow reversal, pulmonary hypertension, and tricuspid regurgitation are vital to assess the severity of MR. LV dilatation is a crucial indicator of progression in asymptomatic insufficiency, and LV-end systolic diameter monitoring is a marker of surgical intervention (1).
Intraoperatively 2D and 3D TOE is suggested to facilitate surgical repair or replacement of the valve (1).
Incidence
MVP prevalence is 2–3%. MVP is the leading cause of MR in developed countries (8,9). The prevalence of MVP in the SCD victims is 2.4% reported by Freed and colleagues in the general population in the Framingham study (8).
Pathophysiology
Different pathological processes can cause prolapse of the mitral valve, such as rheumatic heart disease, endocarditis, Marfan syndrome, and ischemic heart disease, but degenerative MVP attributes especially to a specific gamut of primary lesions (1). These are the fibroelastic deficiency (FED) and Barlow syndrome. FED is a fibrillin deficiency that causes chordal rupture. The annular size is normal, and the mitral valve leaflets are thinned. Patients with Barlow syndrome are typically young individuals. Myxomatous degeneration may lead to mitral annulus calcification and dilatation with thickened leaflets (1).
The presence of a dilated LV in severe MR may imply a period of LV remodeling. In acute primary MR, afterload can decline in the beginning because of the altered route for ejection. With LV volume overloading though, the rather thin-walled LV may enlarge and become hypertrophic. Therefore, the afterload in chronic compensated MR will be normal and increased in chronic decompensated MR (1). Remodeling of the LV can allow MR to be tolerated with no significant symptomatology by enhancing the stroke volume. Development of heart failure and probably cardiac death can manifest rapidly, due to the presence of myocardial dysfunction and sympathetic activation (1). LV remodeling has been correlated with the manifestation of ventricular arrhythmias (1).
Causes of SCD
Ventricular and supraventricular arrhythmias are linked with complications of MVP (1,7). MVP patients have a prevalence of ventricular arrhythmias as high as 34% with premature ventricular contractions (PVCs) as the most common pattern (66% of cases) (10). Moderate-to-severe MR has been demonstrated to be an independent risk factor for generating arrhythmias (1,11). Early repolarization has also been associated with MVP (1). QT dispersion has been documented in MVP and may have a potential role in arrhythmogenesis (8,12).
Valve leaflet dumping in diastole or traction on papillary muscles could serve as a mechanical trigger for ventricular arrhythmias. Redundant and thickened leaflets have been identified as a risk factor for SCA in MVP (8). Endocardial friction lesions in the left ventricle may serve as a focus of arrhythmias as well (8). Some pathology studies have indicated that a cardiomyopathic process accompanying MVP or the autonomic nervous system dysfunction may play a role (8,13,14).
Sriram et al. described a malignant subset group of MVP patients at increased risk of sudden death in a retrospective study. The malignant “phenotype” is characterized by young women with bileaflet MVP, biphasic or inverted T waves in the inferior leads, and frequent complex ventricular ectopic activity with documented ventricular bigeminy or ventricular tachycardia (VT) and PVC configurations of outflow tract alternating with papillary muscle or fascicular origin (15-17).
Just to add to the confusion, Nordhues et al. in a retrospective study of 18,676 patients showed that even though bileaflet MVP is associated with VT, it is not associated with a greater risk of cardiac arrest/ventricular fibrillation or implantable cardioverter defibrillator (ICD) implantation. Paradoxically, bileaflet MVP is associated with a better survival compared to single-leaflet MVP (18,19).
In a study of patients with VT and no history of heart disease, MVP was seen in 25% of cases, and these cases were characterized by increased endomyocardial fibrosis (8). Wilde and colleagues performed detailed mapping studies on a patient with MVP and VT and concluded that delayed afterdepolarization-induced triggered activity was the mechanism, with stretch and fibrosis of the papillary muscles contributing to the origin of the arrhythmia (8).
The genetic substrate has also been linked with MVP and SCD. Missov et al. reported a case of SCD due to a novel disease, causing mutation in the SCN5A gene encoding the cardiac sodium channels in a patient with myxomatous mitral valve disease and flail posterior leaflet (20). The mutation was a non-long QT associated mutation (20).
Towbin et al. reported an association between left ventricular noncompaction, sinus node disease, MVP, ventricular arrhythmias and SCD (21). The heterogeneous phenotype is a result of HCN4 mutation. Six families were identified with a history of ventricular arrhythmias, syncope and SCD (21).
Primary spontaneous rupture of the chordae is the most recognized complication of MVP. The chordal rupture may lead to acute MR and cardiogenic pulmonary edema (22). Intramyocardial small vessel disease is associated with SCD. Fibromuscular dysplasia, one type of small vessel disease, has been found by pathologic examination more frequently in MVP than in the controls (75% vs. 25%). This type is linked to fibrosis of the basal interventricular septum in MVP cases (23).
Table 1 summarizes the mechanisms of SCD in MVP patients (1,8,10,15-17).
Risk of SCD
Patients with MVP and SCD are usually young with few cardiovascular risk factors (8).
Myxomatous degeneration of valve leaflets and other structures of the valve apparatus and the degree of its severity, in particular, has been found to be the major risk factor in arrhythmia development (24).
Cardiac magnetic resonance (CMR) has demonstrated a correlation between MVP and papillary muscle fibrosis (1,7,10,25,26). Basso et al. studied 650 young patients and showed that CMR could detect LV late gadolinium enhancement (LGE) in MVP patients with complex ventricular arrhythmias. Histological examination showed myocardial fibrosis of the LV in SCD population, which was verified in the clinical aspect of the study, with LGE findings at contrast-enhanced CMR in arrhythmogenic MVP patients, closely overlapping the histopathological evidence found in the SCD population (13).
Sheppard et al. reported pathological findings of LV myocardial fibrosis in 46 (74%) patients, affecting one or both papillary muscles and the adjacent LV wall. Myocardial fibrosis was principally localized within the posteromedial papillary muscle and the inner wall of the adjacent posteroinferior wall. Furthermore, the anterolateral papillary muscle and the adjacent anterolateral wall of the LV were mostly affected. The replacement and interstitial type of myocardial fibrosis with subendocardial and mid-mural distribution, including the trabeculae, but never transmural, was consistent with the findings of Basso et al. (25,27). The majority (74%) of the patients died at rest or during sleep (25,27).
Perazzolo et al. investigated 36 (27 female patients; median age: 44 years) arrhythmic MVP patients with LV LGE on CMR and no or trivial MR, and 16 (6 female patients; median age: 40 years) MVP patients without LV LGE (28). Mitral annulus disjunction and a higher prevalence of auscultatory mid-systolic click were correlated with patients with LV fibrosis. Mitral annulus disjunction is a constant component of arrhythmic MVP with LV fibrosis (28). The excessive leaflet mobility caused by posterior systolic curling accounts for a mechanical stretch of the inferobasal wall and papillary muscles, that leads to myocardial hypertrophy and scarring. These mitral annulus abnormalities, together with the auscultatory mid-systolic click, may identify MVP patients who would need arrhythmic risk stratification (28).
Studies involving signal-averaged electrocardiogram (ECG) showed an increased frequency of late potentials in MVP patients, but without any risk prediction. Programmed electrical ventricular stimulation also failed to identify high-risk individuals (8).
Table 2 summarizes the studies investigating the high-risk features for SCD in MVP patients.
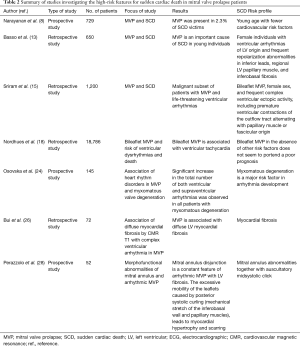
Full table
Mitral valve repair
A study by Naksuk et al. examined the effect of mitral valve surgery on ventricular ectopy burden in thirty-two bileaflet MVP patients. Ventricular ectopy burden was unchanged after mitral valve surgery. Only younger patients had a greater reduction in ventricular ectopy, suggesting that early surgical intervention could modify the underlying electrophysiologic substrate (29).
A retrospective analysis of eight patients with malignant MVP by Vaidya et al. showed that surgical repair of bileaflet MVP alone was associated with a reduction in malignant arrhythmia and appropriate shocks (30).
Hosseini et al. described two cases of MVP patients and refractory ventricular arrhythmias. Both of them had severe MR and underwent mitral valve repair surgery. The arrhythmia burden disappeared after the surgery (31).
Catheter ablation
Syed et al. investigated 14 patients with bileaflet MVP. The patients were subjected to electrophysiological study. The study showed that ablation of clinically dominant ventricular ectopy foci improves symptoms and reduces appropriate ICD shocks (17). Syed et al. established the Purkinje system as an arrhythmogenesis origin in bileaflet MVP (17).
Discussion
The MVP patient with ventricular arrhythmias at increased risk of SCD is generally a young female with a mid-systolic click, a bileaflet MVP, T wave electrocardiographic abnormalities on inferior precordial leads, and right bundle branch block or polymorphic ventricular arrhythmias on ECG (13,17).
The American Heart Association/European Society of Cardiology guidelines for ventricular arrhythmias and SCD have no distinctive criteria for predicting or assuming SCD secondary to MVP (1,2), neither do they have specific recommendations for the management of ventricular arrhythmias or SCD in mitral valvular heart disease (1,2,16).
LV scar burden in targeted areas subjected to larger mechanical stress is the substrate of electric instability in arrhythmogenic MVP and endorse a role for LGE CMR for risk stratification in a certain group of patients (25,27). Fibrosis of the papillary muscles and inferobasal LV segment, indicating a myocardial stretch by the leaflet that prolapses, is the structural hallmark and is associated with ventricular arrhythmias origin (13).
Sheppard et al. autopsy series verified the findings of Basso et al. and suggested that, in the subgroup of patients with MVP with increased-risk clinical indications and mainly complex ventricular arrhythmias, LGE CMR can play a crucial role in the risk stratification assessment and management of these patients (25).
The struggle against SCD must combine primary and secondary prevention methods. There are cardiac causes of SCD that either remain unidentified or, if found, do not still have specific guidelines for management (27). We should be aware that most MVP patients die suddenly at rest or during sleep at home (27).
Screening programs, technological availability (ECG, echocardiography) and cost are impediments for a wide application of primary prevention. Basso et al suggest CMR, treadmill exercise test, and serial Holter monitoring for risk stratification strategy (27). The expected cost of this approach to detect an increased-risk patient and implant an ICD will be $2.3 to $10.6 million per life saved if we choose to screen only patients referred to the hospital after being clinically diagnosed with MVP. However, it would escalate to $277 to $281 million if we begin utilizing echocardiography as the standard screening tool and advance to CMR, exercise tolerance testing, and Holter monitoring when an MVP diagnosis is established (32). Nonetheless, if MVP had been found randomly or in screening (such as relatives of patients with SCD or athletes), it may be simpler to reevaluate suggesting a strategy for those patients depending on distinct risk criteria. An ICD implantation is not without risk also, particularly for young individuals. Electrophysiology study, CMR, and echocardiography may be essential in determining the appropriate group (1).
The potential of biomarkers and genetic studies to determine high-risk individuals is likely to be tested in the future, especially with the recent findings related to familial clustering (8,20,21).
Abbadi et al. emphasized the role of mitral valve repair in the treatment of ventricular arrhythmias resulting from malignant MVP. A female MVP patient with persistent arrhythmias and symptoms and no surgical criteria underwent mitral valve repair. Three years postoperatively the patient was disease-free. The case illustrates the need for high vigilance in the patients with malignant features of MVP, to prevent SCD and also a potential treatment option (33).
Large prospective studies should be designed to evaluate the role of imaging and genetic evaluation in risk stratification for SCD in these challenging groups of patients, as also the effectiveness of antiarrhythmic drug therapy, catheter ablation of targeted areas and surgery in managing ventricular arrhythmia in patients with MVP.
Conclusions
MVP is a common condition in the general population and is often encountered in asymptomatic individuals. The existing literature continues to generate significant controversy regarding the association of MVP with ventricular arrhythmias and SCD.
Echocardiography and CMR can accurately delineate the structure and function of the mitral valve. Fibrosis of the papillary muscles and the inferobasal LV segment is the structural hallmark and is associated with ventricular arrhythmias origin. CMR can identify the patients with arrhythmia substrate.
Early echocardiography and CMR are essential, as is a better understanding of the possible electrophysiological processes of primary arrhythmogenesis.
The results of our present analysis lead to the conclusion that MVP is an underrated cause of arrhythmic SCD. The subset of patients with malignant MVP who may be at greater risk for SCD is characterized by young women with bileaflet MVP, biphasic or inverted T waves in the inferior leads, and frequent complex ventricular ectopic activity with documented ventricular bigeminy or VT and PVC configurations of outflow tract alternating with fascicular origin or papillary muscle.
Early mitral valve repair and catheter ablation can reduce the arrhythmia burden, but data derive from small single-center studies.
We do believe that the role of imaging and genetic evaluation in risk stratification for SCD in MVP patients deserves further experimental investigation and large-scale prospective randomized clinical trials. Extensive studies must also be conducted to assess early mitral valve repair and catheter ablation as treatment strategies.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Ahmed M, Roshdy A, Sharma R, et al. Sudden cardiac arrest and coexisting mitral valve prolapse: a case report and literature review. Echo Res Pract 2016;3:D1-8. [Crossref] [PubMed]
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793-867. [Crossref] [PubMed]
- Brzyzkiewicz H, Wałek P, Janion M. Sudden cardiac arrest in ventricular fibrillation mechanism as a first manifestation of primary mitral valve prolapse. Przegl Lek 2012;69:1306-8. [PubMed]
- Franchitto N, Bounes V, Telmon N, et al. Mitral valve prolapse and out-of-hospital sudden death: a case report and literature review. Med Sci Law 2010;50:164-7. [Crossref] [PubMed]
- Anders S, Said S, Schulz F, et al. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Sci Int 2007;171:127-30. [Crossref] [PubMed]
- Rajani AR, Murugesan V, Baslaib FO, et al. Mitral valve prolapse and electrolyte abnormality: a dangerous combination for ventricular arrhythmias. BMJ Case Rep 2014;2014. pii: bcr2014205055.
- van der Wall EE, Schalij MJ. Mitral valve prolapse: a source of arrhythmias? Int J Cardiovasc Imaging 2010;26:147-9. [Crossref] [PubMed]
- Narayanan K, Uy-Evanado A, Teodorescu C, et al. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm 2016;13:498-503. [Crossref] [PubMed]
- Delling FN, Vasan RS. Epidemiology and Pathophysiology of Mitral Valve Prolapse. Circulation 2014;129:2158-70. [Crossref] [PubMed]
- Noseworthy PA, Asirvatham SJ. The Knot That Binds Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015;132:551-2. [Crossref] [PubMed]
- Turker Y, Ozaydin M, Acar G, et al. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging 2010;26:139-45. [Crossref] [PubMed]
- İmamoğlu EY, Eroğlu AG. QT dispersion and ventricular arrhythmias in children with primary mitral valve prolapse. Turk Pediatri Ars 2016;51:135-41. [Crossref] [PubMed]
- Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015;132:556-66. [Crossref] [PubMed]
- Bohora S. Mitral valve surgery: Does it really decrease ventricular arrhythmia in patients with mitral valve prolapse? Indian Pacing Electrophysiol J 2016;16:185-6. [Crossref] [PubMed]
- Sriram CS, Syed FF, Ferguson ME, et al. Malignant Bileaflet Mitral Valve Prolapse Syndrome in Patients With Otherwise Idiopathic Out-of-Hospital Cardiac Arrest. J Am Coll Cardiol 2013;62:222-30. [Crossref] [PubMed]
- Lancellotti P, Garbi M. Malignant Mitral Valve Prolapse. Circ Cardiovasc Imaging 2016;9:e005248. [Crossref] [PubMed]
- Syed FF, Ackerman MJ, McLeod CJ, et al. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ Arrhythm Electrophysiol 2016;9:e004005. [Crossref] [PubMed]
- Nordhues BD, Siontis KC, Scott CG, et al. Bileaflet Mitral Valve Prolapse and Risk of Ventricular Dysrhythmias and Death. J Cardiovasc Electrophysiol 2016;27:463-8. [Crossref] [PubMed]
- Al-Khatib SM. The Risk of Sudden Cardiac Death in Mitral Valve Prolapse: Are All Patients Created Equal? J Cardiovasc Electrophysiol 2016;27:469-70. [Crossref] [PubMed]
- Missov E, Cogswell R. Sudden Cardiac Death, Mitral Valve Prolapse, and Long QT Syndrome. Am J Med 2015;128:e37-8. [Crossref] [PubMed]
- Towbin JA. Ion channel dysfunction associated with arrhythmia, ventricular noncompaction, and mitral valve prolapse: a new overlapping phenotype. J Am Coll Cardiol 2014;64:768-71. [Crossref] [PubMed]
- Hickey AJ, Wilcken DE, Wright JS, et al. Primary (spontaneous) chordal rupture: relation to myxomatous valve disease and mitral valve prolapse. J Am Coll Cardiol 1985;5:1341-6. [Crossref] [PubMed]
- Veinot JP, Johnston B, Acharya V, et al. The spectrum of intramyocardial small vessel disease associated with sudden death. J Forensic Sci 2002;47:384-8. [Crossref] [PubMed]
- Osovska NY, Kuzminova NV, Knyazkova II. Cardiac arrhythmias in adolescents with mitral valve prolapse and myxomatous degeneration of mitral valve leaflets. Wiad Lek 2016;69:730-3. [PubMed]
- Sheppard MN, Steriotis AK, Sharma S. Letter by Sheppard et al Regarding Article, “Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2016;133:e458. [Crossref] [PubMed]
- Bui AH, Roujol S, Foppa M, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017;103:204-9. [Crossref] [PubMed]
- Basso C, Marra MP, Rizzo S, et al. Response to Letters Regarding Article, “Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2016;133:e460. [Crossref] [PubMed]
- Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [Crossref] [PubMed]
- Naksuk N, Syed FF, Krittanawong C, et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J 2016;16:187-91. [Crossref] [PubMed]
- Vaidya VR, DeSimone CV, Damle N, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol 2016;46:137-43. [Crossref] [PubMed]
- Hosseini S, Rezaei Y, Samiei N, et al. Effects of mitral valve repair on ventricular arrhythmia in patients with mitral valve prolapse syndrome: A report of two cases. Int J Cardiol 2016;222:603-5. [Crossref] [PubMed]
- Providencia R, Lambiase PD. Letter by Providencia and Lambiase Regarding Article, “Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2016;133:e459. [Crossref] [PubMed]
- Abbadi DR, Purbey R, Poornima IG. Mitral valve repair is an effective treatment for ventricular arrhythmias in mitral valve prolapse syndrome. Int J Cardiol 2014;177:e16-8. [Crossref] [PubMed]



