The absence of effect of ganglionated plexi ablation on heart rate variability parameters in patients after thoracoscopic ablation for atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. Several studies have documented the superiority of catheter ablation (CA) over treatment with anti-arrhythmic drugs (AADs) (1,2). Therefore, CA is recommended in symptomatic AF patients, especially after failure of AADs (3). Pulmonary vein isolation (PVI) is the cornerstone of all ablation techniques performed in AF patients. The success rate of PVI has been described as between 60–70% in paroxysmal AF patients, although less in patients with persistent or long-standing persistent AF (1,2). Due to limited efficacy of PVI, other ablation techniques have been developed and introduced.
The autonomic nervous system (ANS) and ganglionated plexi (GP) play an important role in the initiation and maintenance of AF (4). GP are located in several typical areas (i.e., in pericardial fat of the left atrium) (5,6). Endocardial CA of the GP has been shown to increase the efficacy of the ablation for paroxysmal, as well as persistent AF (4,7,8). Although it is difficult to assess the effect of GP ablation, it can be done using heart rate variability (HRV) analysis. HRV is a non-invasive and highly reproducible technique (9,10).
The treatment of patients with persistent or even long-standing persistent AF still presents an electrophysiological (EP) challenge. Recently, minimally invasive thoracoscopic procedures have been introduced. According to a recently published review, the efficacy of surgical thoracoscopic ablation (TA) is relatively high, although it must be noted that patients with persistent or long-standing persistent AF were the primary patients enrolled in the individual studies that were included in the review (11). Currently the goal of TA is to create a box lesion around all pulmonary veins (either as a block, using one single linear catheter, or by clamping all PVs and making connection lines between the superior and inferior PVs). Epicardial TA routinely, although not intentionally, involves areas where the GP are located and the coincidental ablation of the GP could be one of the reasons for the relatively high efficacy of these procedures. Whether the GP are really “coincidentally” damaged during epicardial TA has not been properly studied.
We hypothesized that TA ablations could be associated with unintentional damage or ablation of the GP. Therefore, additional targeted GP ablation during a subsequent CA might result on only minimal changes in HRV parameters. The aim of the study was to assess the effect of GP ablation on HRV parameters (determined before and after CA) in patients having undergone a previous TA, and compare them to the HRV of GP ablation patients without a previous TA.
Methods
Patient population
Patients with paroxysmal, persistent, or long-standing persistent AF were enrolled in the study, which defined AF according to the current AHA/ACC/HRS guidelines (3). The study was performed as a prospective, observational study, written informed consent was obtained from each participant. The study was approved by institutional ethics committee of University hospital Kralovske Vinohrady (No. EK-VP/28/3/2015). Exclusion criteria included the absence of sinus rhythm before and after the EP procedure, administration of any AADs during the procedure, or the need for DC version during the procedure.
Group I (Hybrid group) consisted of patients with persistent or long-standing persistent AF recommended for a two-staged, hybrid ablation. In this group of patients, the hybrid ablation was offered based on our institutional program of hybrid ablation of patients with persistent and long-standing persistent AF patients (12).
The major exclusion criteria for the hybrid procedure included significant structural heart disease or indication for open heart surgery (detailed inclusion, exclusion criteria and follow-up were previously published) (12). An additional exclusion criterion of in the present study was the absence of sinus rhythm at the beginning of the EP study: in such a situation, HRV analysis cannot be done. The ablation protocol and follow-up of patients is described below.
Group II (GP group) consisted of patients with paroxysmal or persistent AF, which were indicated for PVI as a routine procedure for treatment of AF according to the current AHA/ACC/HRS guidelines (3). The inclusion criteria were paroxysmal or persistent AF and a signed informed consent, the major exclusion criteria included significant valvular disease, severe left ventricular dysfunction, thyrotoxicosis, left atrial diameter >60 mm, and, as in the other groups, absence of sinus rhythm at the beginning of the EP procedure.
Ablation protocols: in both groups, the GP ablation was done in a similar manner, i.e., location and energy delivery. The ablation protocol of each group is described below.
Hybrid group—surgical part
The hybrid approach consisted of a unilateral (right side) epicardial thoracoscopic procedure, followed by endocardial CA. The protocol was previously described (12).
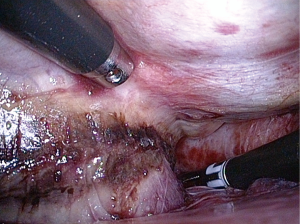
The surgical part was performed under general anesthesia, single-lung ventilation, and a right thoracoscopic approach. After opening the pericardium, the transverse and oblique sinuses were bluntly dissected using both electrocoagulation and endoscopic instruments. The layer of pericardial fat was also dissected. Then the COBRA Fusion™ 150 (Estech, an AtriCure® Company, San Ramon, CA, USA) ablation catheter was positioned around the PVs using two special introducers. The position of the catheter (encircling all four PVs) was checked repeatedly, both visually and with transesophageal echocardiography (TEE), mainly to confirm the correct position under the left atrial appendage (LAA). The position of the COBRA Fusion catheter, during surgical ablation (and its relationship to common localization of GPs), is shown in Figure 1. Suction was then started and set to a minimum of ‒500 mmHg, what assured stable contact between atrial tissue and the catheter᾿s electrodes. Using the COBRA Fusion catheter, radiofrequency (RF) energy was applied, both in the bipolar and unipolar mode, using a temperature-controlled energy application setting of 70 °C and 60 seconds per cycle. The first cycle was performed in both modes, then the catheter was moved circumferentially and the second cycle performed. The visible part of the lesion was checked and a second, overlapping, ablation was performed if the lesion did not look continuous. This occurred mainly in the area of the interatrial groove and was more often required in patients with a large LA. In other words, during the ablation, at least 120 seconds of unipolar and 120 seconds of bipolar RF energy was applied at each spot of the intended “box-lesion” line. If the patient remained in AF after the ablation, a direct current cardioversion was performed.
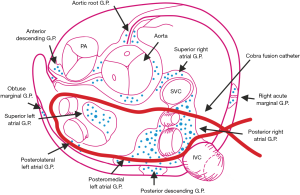
The COBRA Fusion catheter includes six electrodes, each 25-mm long. Contact with atrial tissue is assured through suction of the tissue into the catheter, between the electrodes. Since the catheter is 5 mm wide and 5 mm deep, a relatively large volume of tissue is sucked into the catheter and exposed to RF energy. The size of the lesion created by the COBRA ablation catheter is 15 mm, which is large enough to be visually inspected (Figure 2). During the procedure, 2–3 ablation cycles were carried out. After each ablation cycle, the COBRA catheter is moved, i.e., widening the size of the final lesion line. To insure good-contact with the atrial myocardium and to ablate the maximum amount atrial myocardial tissue and the least amount of epicardial fat, we dissected and removed as much epicardial fat as possible from the area of the intended lesion. This was done in areas which are visible during the surgery, i.e., the left atrium (LA) roof and area of the interatrial groove, which are also common GP locations. During the TA, no specific GP mapping was carried out; the goal of the procedure was to isolate all four PVs and the posterior wall of the left atrium using a “box lesion block.”
Hybrid group—EP part
The EP study and CA part of the procedure were performed 2–3 months after the surgical procedure and was previously described in detail (12). The aim of the EP study was to (I) verify (and complete, if needed) the box isolation; (II) perform an ablation of the GP in the LA, and, in the end; (III) perform an ablation of the cavotricuspid isthmus. In patients with regular supraventricular tachycardia, an addition goal was to map and ablate the ongoing arrhythmia.
All procedures were performed using the CARTO 3 (Biosense Webster) mapping system and with a 3.5 mm irrigated-tip CARTO catheter (ThermoCool Smart-Touch, Biosense-Webster, Inc.). RF lesions were delivered with a contact force >5 g, RF energy between 20–35 W, and a temperature limit of 43 °C. The procedure was previously described in detail (12). Based on the work of Pokushalov et al. (4,7), the last step in the PVI was the GP ablation, which was done empirically at presumed sites of GP clustering. In brief, four areas of the LA were ablated: (I) superior and posterior to the left superior pulmonary vein (LSPV) (1–2 cm outside the ostium of the LSPV and in the area between the superior aspect of the PV antrum and the posterior LA wall); (II) inferior and posterior to the left inferior pulmonary vein (LIPV); (III) anterior to the right superior pulmonary vein (RSPV) (1–2 cm from the RSPV to the superior-anterior LA wall); and (IV) below the right inferior pulmonary vein (RIPV) (1–2 cm inferior to the RIPV). The ablation of the ganglionated plexuses (GPs) was done in this aforementioned sequence in all patients. Each GP received at least 5 RF applications (30 s, 20–35 Watts according to the ablation site) with a mean RF time of 4.6±2.3 min. Next the sheath was withdrawn to the right atrium, a 20-polar hallo catheter was added, and an ablation of the cavotricuspid isthmus was performed.
Successful procedures had to meet the following criteria: (I) complete box isolation (verified by exit and entrance block, all four PVs were isolated and roof and bottom line is complete); and (II) complete cavotricuspid isthmus line.
CA—GP group
All EP procedures were performed using a CARTO 3 mapping system and a LabSystem Pro (Boston Scientific) EP recording system, using a decapolar catheter inserted into the coronary sinus (Dynamic, Boston Scientific). Two trans-septal punctures were done under intracardiac echocardiography (ICE) (AcuNav, Siemens) guidance, one for the circular mapping (Lasso) catheter and the other for CARTO ablation catheter. After creating an anatomical CARTO map of the left atrium, the borders of all four PVs were visualized using ICE and marked on the CARTO map. Next, all four PVs were isolated using point-by-point radiofrequency ablation with a 3.5 mm irrigated-tip CARTO catheter (ThermoCool Smart-Touch, Biosense-Webster, Inc.). RF ablation settings were similar to those used in the Hybrid group (i.e., contact force of >5 g, a temperature limit of 43 °C, RF energy delivery of 25–35 Watts on the anterior wall, and 20–25 watts on the posterior wall). In the majority of cases, the left veins were isolated first using one wide circle, but without touching the carina between them. Next, the right pulmonary veins were separately isolated. The isolation of each vein was confirmed, using Lasso catheters, by the presence of an entrance and exit block. The GP ablation was carried out in the same manner as described in the hybrid approach (EP-part of the paragraph above). The operation was successful if all four PVs were isolated at the end of the procedure.
HRV assessment
HRV, as an indicator of ANS function, was evaluated using standard techniques before and after the EP procedure in all patients in both groups. HRV expresses the degree of variation in the intervals between QRS complexes during normal sinus rhythm. Data for analyses were collected prospectively. An electrocardiogram (ECG) was recorded before the procedure (while the patient was on the EP table in the EP lab just before the groin puncture) and again after the procedure (after all catheters had been withdrawn, although sheaths were still in the inferior vena cava). The LabSystem Pro EP recording system (Boston Scientific) was used for signal recording. Five-minute-long signals (before and after the procedure) from each patient were exported to ‘.txt’ files. These ‘.txt’ files were prepared in a specific format using appropriate software for further analysis using Kubios HRV analysis software (University of Eastern Finland) (13). All the analyses were done offline after the procedures; however, all ECG recordings were collected prospectively.
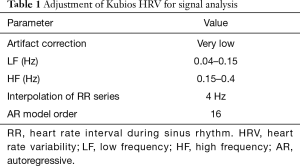
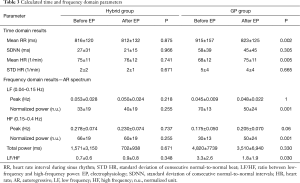
We made an adjustment to the Kubios HRV software, which is shown in Table 1. All signals were checked and any incorrect detection of QRSs were manually corrected. Premature ventricular beats and premature atrial beats were excluded. The time-domain parameter, which was the mean heart rate or standard deviation of instantaneous heart values, was evaluated. Spectral analysis of 5-min ECG segments was performed. The normalized values of heart work at low frequencies (LFs) and high frequencies (HFs) were investigated. The autonomic balance was assessed by the spectral LF to HF ratio (LF/HF ratio).
Full table
Statistical analysis was done using SigmaStat 4.0 software (Systat Software, Inc.). Data were tested for normal distribution using the Shapiro-Wilk test. If the normality test was passed, the differences in the mean value of the parameters were tested using the paired t-test. If the normality test failed, the Wilcoxon Signed Rank test was used. P values ≤0.05 were considered significant.
Results
Patient’s characteristics
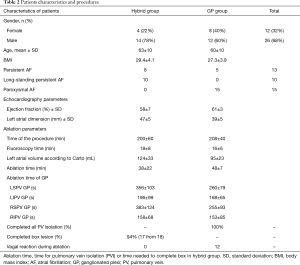
All patients in this study underwent the EP part of the procedure between January 2014 and November 2016. Thirty-eight patients were enrolled in the study. Group I (Hybrid) consisted of 18 patients (14 men, 4 women), Group II (GP) consisted of 20 patients (12 men, 8 women). The patient characteristics and procedures are shown in Table 2. During the procedure, all patients were slightly sedated, using midazolam (approx. 2–5 mg/procedure) and fentanyl (approx. 0.2–0.6 mg/procedure); the sedation was similar in all groups.
Full table
In Group I (Hybrid), 8 patients suffered from persistent AF and 10 patients from long-standing persistent AF. In Group II (GP), 5 patients suffered from persistent AF and 15 patients suffered from paroxysmal AF. Left atrial dimensions were greater in the Hybrid group compared to GP group. This difference was confirmed by left atrial volumes measured using CARTO (Table 2).
Ablation characteristics
In the Hybrid group, successful ablation (i.e., box lesion completeness) was achieved in 94% of patients (17 of 18 patients had a completed box lesion at the end of the EP study). The box lesion was complete in 8 of 18 (44%) patients at the beginning of the procedure (i.e., after TA only). The remaining 10 patients (56%) required completion of the box lesion with a CA. The mean time of RF energy needed to complete the box was 38±22 min. In the GP group, PVI was successful in all patients (i.e., all four veins were isolated in all patients). The mean time of RF energy delivery was 48±7 min.
Regarding the GP ablation, the ablation delivery was similar in both groups. The time of energy delivery on the ganglion superior-posterior to LSPV was 5.9±1.7 min in the Hybrid group and 4.3±1.3 min in the GP group; on the ganglion inferior and posterior to the LIPV was 3.3±1.7 min in the Hybrid group and 2.7±1.1 min in the GP group, on the ganglion anterior to RSPV was 6.4±2.1 min in the Hybrid group and 4.3±1.5 min in the GP group, and finally, on the ganglion below the RIPV, the RF time was 2.6±1.1 min in the Hybrid group and 2.6±1.4 min in the GP group [all P= not significant (n.s.)].
Vagal responses
Positive vagal responses [VR, defined by Qin (14) as a >20% decrease in heart rate during sinus rhythm)] during ablation was not observe in any of the patients in the Hybrid group. However, VR were present in 12 (60%) patients in the GP group.
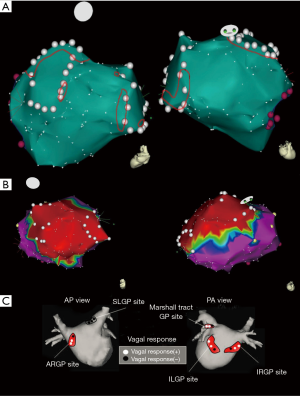
The mean number of VR per patient during ablation was 3.8±2.8 VRs (range, 1–10 VR) in the GP group. Eleven patients (92%) experienced VR during ablation anterior to the LSPV, 2 patients (17%) posterior to the LIPV, 5 (42%) anterior to the RSPV, and 7 (58%) anterior to the LIPV. Most VRs occurred during the PVI procedure itself. VR during targeted ablation of GP were observed in only 3 patients (25%) in the GP group. Typical areas with vagal responses observed during ablations are shown in Figure 3.
HRV
Five-minute-long ECG recordings of all patients obtained before and after the EP procedure were analyzed. The results are shown in Table 3. The most important parameter among the time-domain results was heart rate (HR). A statistically significant increase in the HR was observed in the GP group (68±12 bpm before EP/75±11 bpm after EP, P=0.005); however, this was not seen in the Hybrid group. When analyzing only patients with persistent AF (5 patients) in GP group, similar results relative to the changes in the HR were present: i.e., 66±7 bpm at the beginning of the procedure and 74±9 bpm at the end of the procedure (due to small number of patients, no statistical analysis was done).
Full table
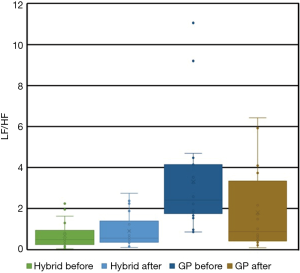
Regarding the analysis of the frequency-domain, the LF and HF parameters were analyzed. The results of peak frequency, as well as the HF- and LF-domain parameters are shown in Table 3. There was no change in the normalized power in HF or LF component in the Hybrid group. In contrast, the normalized power in the LF part decreased significantly after the procedure, compared to values before the procedure, in the GP group. Additionally, in the GP group, the normalized power in the HF component increased after the procedure compared to values before the procedure. Similarly, no change in the LF/HF ratio was present in the Hybrid group (before 0.7±0.6 and after 0.9±0.8, P=n.s.); however, the LF/HF ratio decreased significantly in the GP group (3.3±2.6 before and 1.8±1.9 after the procedure, P=0.03, see Figure 4). Regarding the subgroup of persistent AF patients in the GP group (5 patients), there was a decrease of the LF/HF ratio in three patients, and no change in the remaining two patients. However, this subgroup was too small for statistical evaluation.
Discussion
In our study, significant differences in HRV parameters, compared before and after CA, were present in patients undergoing PVI and GP ablation, who were without a history of a previous TA. On the other hand, no changes in HRV parameters, before and after endocardial GP ablation, were found in AF patients with a history of a previous TA.
Epicardial ablation has become a widely-used technique for AF ablation, especially in patients with persistent and long-standing persistent AF. The catheter used in our study was constructed to encircle the posterior left atrium anterior to the pulmonary veins—once positioned around the left atrium, it covers the roof aspect of the left atrium anterior and superior to the superior veins, as well as the region inferior to the veins. The locations of GP vary between patients; however, in the majority of patients, five GP have been described: right (superior) anterior (located anterior to the RSPV), left superior (located anterior to the LSPV), left inferior (located inferior and medial of the LIPV), and right inferior (located around the RIPV) (5). Thus, comparing the location of GPs relative to the ablation line made by the COBRA catheter, the catheter approximately covers the area most often associated with GPs, which means that, although not intentional, epicardial ablations made using the COBRA catheter likely also ablate the most common GP sites. No mapping for location was done either during TA, or endocardial CA. However, as shown in endocardial CA studies, an anatomical ablation performed empirically (i.e., at presumed GP sites) was associated with similar results compared to ablations driven by meticulous GP localization using high-frequency stimulation (15). This can be explained by the GP, in the majority of patients, being in nearly the same locations. Although our thoracoscopic approach was not intentionally aimed to ablate GPs (no maneuvers, such as high-frequency stimulation, were done to locate the GPs accurately), the ablation line of the COBRA catheter, nonetheless, covered the locations where GP are mainly found. Thus, one can expect that the ablations using the COBRA linear catheter could lead to GP ablation as well and our findings confirm this expectation. The parameters of HRV were changed significantly during PVI + GP ablation, which was in contrast to hybrid patients, in whom no changes of HRV parameters were present. Not a single vagal response was observed during GP ablation in the hybrid patients, in contrast to the GP group patients, where a vagal response was present in 60%.
The clinical effects of GP ablation are difficult to assess and our study was not designed to assess these effects. Some authors have shown that GP ablation is associated with more favorable outcomes in terms of AF freedom, even in patients with persistent AF (4,7), while other reports have failed to confirm these benefits (16). Our cohort was too small to analyze the clinical follow-up and the importance of GP ablation. The clinical results of hybrid ablation seem to be promising and not easily explained by the box lesion itself. In catheter based studies, the box lesion, i.e., isolation of the whole posterior wall by PVI, plus the additional of a roof line and inferior line, was not found to be superior compared to PVI alone (17,18).
Despite several observational and randomized clinical studies regarding the clinical effect of GP ablation, few studies have been published that address the exact effect of GP (or PVI with and without concomitant GP ablation) ablation on HRV parameters.
Ketels et al. (19) showed that circumferential PVI (without additional GP ablation) induced an acute acceleration in the heart rate. They reported that the HR at the end of the procedure (62±9 bpm) was significantly higher compared to the HR before the procedure (54±8 bpm). This finding was very similar to that seen in our GP patients, in whom the HR increased from 68±12 (pre-procedure) to 75±11 bpm (post-procedure). Wang et al. (20) demonstrated that HRV parameters (e.g., LF/HF ratio) decreased significantly after both segmental and circumferential PVI; this decrease was similar to that seen in our GP patients. Similarly, Suwalski et al. (21) showed a comparable decrease in LF/HF after epicardial PVI, which was performed during coronary artery bypass grafting in patients with coronary artery disease and concomitant AF. The LF/HF ratio, which is assumed to be an important parameter of sympathovagal balance, decreased significantly in our GP group, which confirmed the effect of ablations on autonomic tone. No change in the LF/HF ratio was present in the Hybrid group. Unfortunately, we couldn’t compare changes in the LF/HF ratio before and after surgical ablation because almost all the patients were in AF at the beginning of the thoracoscopic procedure, thus, no baseline analysis before ablation could be performed. However, the comparison of the LF/HF ratio between the Hybrid and GP group before the endocardial EP procedure showed that the LF/HF ratio was significantly lower in the Hybrid group than in the GP group, even before the procedure.
During a catheter endocardial ablation procedure, the epicardially located GPs are damaged only via conductive heating. On the other hand, a TA affects just the immediate area where GP are located. The width and depth of the COBRA catheter, with a lesion size of 15–20 mm, indicates that the amount of pericardial tissue ablated during TA can be quite large. Although not intentionally, a TA using a COBRA catheter can easily damage GP sites. Therefore, no effect on HRV parameters during targeted GP ablation performed during a subsequent endocardial procedure would be present.
In our report, the two groups were not comparable regarding the type of AF and associated parameters (such as LA size). In our center, patients with persistent or long-standing persistent AF are recommended to hybrid ablation. Moreover, a large percentage of patients with persistent or long-standing persistent AF, who refused a hybrid ablation, and CA ablations, were in AF before the CA; thus, HRV measurements were not possible, which explains the absence of any long-standing persistent AF patients in the GP group.
Theoretically, the absence of long-standing persistent AF patients in the GP group could have affected our results. However, in the literature, positive clinical effects associated with GP ablation in persistent and long-standing persistent AF patients have been described. Pokushalov et al. showed that a GP ablation and additional circumferential isolation of all PV offered a success rate of 59.6% (8), and the acute effect of PVI on HRV parameters was described even in these patients (22). In our patients, the effect of GP ablation on HRV parameters was also similar in the subgroup of GP patients with persistent AF; although due to the small number of patients with persistent AF, no statistical analysis could be done. While we cannot exclude the effect the type of arrhythmia might have on HRV following a GP ablation, the complete absence of any effect of GP ablation among our Hybrid patients in our opinion, cannot be explained by arrhythmia type only, but is understandable in terms of TA-associated incidental GP ablation.
Conclusions
Vagal responses and ANS changes were observed in the GP group. Targeted ablation of GPs in patients subsequent to TA had no effects on any of the measured HRV parameters. TA using a COBRA linear catheter might appears to lead to incidental ablation of the GP as well.
Study limitation
The major limitation of the current study is the small number of patients enrolled in the hybrid group. Only patients with symptomatic persistent and long-standing AF are recommended to have that procedure, therefore, the number of patients was limited. Moreover, only patients who are in sinus rhythm (SR) at the beginning of the EP procedure, could have been enrolled (patients in AF cannot be analyzed). It further decreased the number of available patients. Another limitation was the absence of ECG recordings and HRV analysis of hybrid patients during the surgical part of the procedure. The analysis of ECG before and after surgery could have been very important to assess the effect of surgery on HRV, unfortunately, due to inclusion criteria for hybrid ablation (persistent or long-standing persistent AF), the vast majority of patients were in AF at the beginning of the surgery and therefore this analysis could not have been done. Finally, the heterogeneity of the patients was an issue, with the majority of PVI + GP patients having paroxysmal AF, in whom the effects of PVI could differ. However, the study was completely consistent with our current policies of only recommending hybrid ablation to persistent AF patients, while paroxysmal AF patients were recommended to undergo PVI.
Acknowledgements
Funding: The study was supported by a research grant from the Ministry of Health, Czech Republic, Nr. AZV 16-32478A.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of University hospital Kralovske Vinohrady (No. EK-VP/28/3/2015) and written informed consent was obtained from all patients.
References
- Wilber DJ, Pappone C, Augello G, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010;303:333-40. [Crossref] [PubMed]
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498-505. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:2246-80. [Crossref] [PubMed]
- Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation a randomized clinical trial. J Am Coll Cardiol 2013;62:2318-25. [Crossref] [PubMed]
- Armour JA, Murphy DA, Yuan BX, et al. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997;247:289-98. [Crossref] [PubMed]
- Takahashi K, Okumura Y, Watanabe I, et al. Anatomical proximity between ganglionated plexi and epicardial adipose tissue in the left atrium: implication for 3D reconstructed epicardial adipose tissue-based ablation. J Interv Card Electrophysiol 2016;47:203-12. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Katritsis DG, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm 2013;10:1280-6. [Crossref] [PubMed]
- Pokushalov E, Romanov A, Artyomenko S, et al. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace 2010;12:342-6. [Crossref] [PubMed]
- Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 2004;134:514-22. [PubMed]
- Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043-65. [Crossref] [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2014;45:401-7. [Crossref] [PubMed]
- Osmancik P, Budera P, Zdarska J, et al. Electrophysiological findings after surgical thoracoscopic atrial fibrillation ablation. Heart Rhythm 2016;13:1246-52. [Crossref] [PubMed]
- Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV – Heart rate variability analysis software. Comput Methods Programs Biomed 2014;113:210-20. [Crossref] [PubMed]
- Qin M, Liu X, Jiang WF, et al. Vagal response during pulmonary vein isolation: Re-recognized its characteristics and implications in lone paroxysmal atrial fibrillation. Int J Cardiol 2016;211:7-13. [Crossref] [PubMed]
- Katritsis DG, Giazitzoglou E, Zografos T, et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm 2011;8:672-8. [Crossref] [PubMed]
- Oswald H, Klein G, Koenig T, et al. Cryoballoon pulmonary vein isolation temporarily modulates the intrinsic cardiac autonomic nervous system. J Interv Card Electrophysiol 2010;29:57-62. [Crossref] [PubMed]
- Tamborero D, Mont L, Berruezo A, et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol 2009;2:35-40. [Crossref] [PubMed]
- Chilukuri K, Scherr D, Dalal D, et al. Conventional pulmonary vein isolation compared with the “box isolation” method: a randomized clinical trial. J Interv Card Electrophysiol 2011;32:137-46. [Crossref] [PubMed]
- Ketels S, Houben R, Van Beeumen K, et al. Incidence, timing, and characteristics of acute changes in heart rate during ongoing circumferential pulmonary vein isolation. Europace 2008;10:1406-14. [Crossref] [PubMed]
- Wang K, Chang D, Chu Z, et al. Denervation as a common mechanism underlying different pulmonary vein isolation strategies for paroxysmal atrial fibrillation: evidenced by heart rate variability after ablation. ScientificWorldJournal 2013;2013:569564. [PubMed]
- Suwalski G, Suwalski P, Kalisnik JM, et al. How does successful off-pump pulmonary vein isolation for paroxysmal atrial fibrillation influence heart rate variability and autonomic activity? Innovations 2008;3:1-6. [PubMed]
- Seaborn GE, Todd K, Kevin A., et al. Heart rate variability and procedural outcome in catheter ablation for atrial fibrillation. Ann Noninvasive Electrocardiol 2014;19:23-33. [Crossref] [PubMed]


