Clinical assessment of computed tomography guided radiofrequency ablation in the treatment of inoperable patients with pulmonary tumors
Introduction
Lung cancer is one of the most common malignant tumors in the world (1). In China, the mortality rate of lung cancer has increased by 465% compared with 30 years ago and there were about 0.6 million patients who died from lung cancer each year (2,3). The proportion of the elderly patients continued increasing along with the population ages, which were often accompanied with different kinds of complications and could not tolerate conventional treatment, such as surgery, chemotherapy and radiotherapy. Hence, serious of local treatment methods were developed, such as radiofrequency ablation (RFA), cryoablation, and microwave ablation (4). Among them, only RFA was listed in the US National Comprehensive Cancer Network clinical guidelines on non-small cell lung cancer (NSCLC) (5).
In RFA, high-frequency alternating currents with frequency between 460–480 kHz were used to transform the radiofrequency energy into heat energy. Local temperature could be escalated to 60–100 °C, which lead to the coagulated necrosis of tumor cells (6). RFA was one of the treatments for tumors and it had been accepted as a viable therapeutic option for patients with unresectable liver cancer (7).
Different from liver tissue, lung tissue has several specific features, including an air-bearing organ, rich blood supply and persistent respiratory movement (8). RFA was first reported in treating three cases of lung tumor in 2000 (9). A serious of clinical application of RFA for lung cancer had been reported since then. A series of studies showed satisfactory outcome in the treatment of NSCLC and lung metastases (10-12) while others showed a higher local failure rate in RFA compared with sublobar resection or stereotactic body radiation therapy (4,13). Furthermore, the assessment of quality of life (QoL) is an emerging primary outcome measure for the treatment of lung cancer, but clinical studies which focus on it is limited (14).
In the present study, we retrospectively analyzed clinical characteristics and outcome of lung cancer patients who underwent RFA, with the purpose to enhance our understanding of the feasibility, effectiveness, safety and QoL of RFA for non-surgical patients with pulmonary tumors in China.
Methods
Patients
This study was designed as retrospective analysis of clinical characteristics and outcome of lung cancer patients who underwent RFA. Inclusion criteria were as follows: age greater than 18 years; NSCLC or lung metastasis proved by biopsy; patients rejected or considered unsuitable for radiotherapy, chemotherapy (poor response to treatment) and surgery (patients with poor heart and lung functions; elderly patients); up to three tumors per lung; tumors accessed by percutaneous route. Exclusion criteria were as follows: previous pneumonectomy; patients who had major comorbid medical conditions and considered high-risk for RFA (international normalized ratio more than 1.5; platelet count less than 100×109/L); more than three tumors per lung; pregnant or breast-feeding; active infection; patients with poor compliance. This study was done with the approval of the ethics committee of Nanjing Medical School (No. LLSC2012-08).
Pre-treatment
The following were done during the screening period within 2 weeks before the treatment: physical examination and collection of data on vital signs, demographics, and medical history; radiological imaging for tumor assessment by using enhanced CT; tumor biopsy; electrocardiography; pulmonary function testing. Within one week before treatment, complete blood count, measurement of electrolyte panel, chemistry panel and coagulation panel were also done. Water deprivation, antitussive or sedative was used 2 hours before treatment.
Treatment
RFA was done by using 250W generator (RITA Medical Systems Model 1500X), which could use power-controlled mode and temp-controlled mode based on impedance. Multi-slice spiral CT was used for guidance and scanning parameters were as following: (120 kV, 200 mA; collimation: 5 mm; pitch: 1.25).
After localization of the nodules on the preview, the skin entry site that allowed the shortest and safest path which avoided bullae, interlobar fissures, or pulmonary vessels was chosen. The RFA probe was placed into the targeted nodules and tines were deployed to create an ablation zone. The ablation protocol was always planned with the aim to destroy the visible nodules plus at least a 0.5–1 cm safety margin around the nodules. Small nodules (greatest diameter <5 cm) were ablated by using a single RFA probe once, while large nodules (greatest diameter >5 cm) must be ablated by one or two RFA probes more than once. The power of RFA gradually increased from 5W with single RFA probe and 20W with two RFA probes. The temperature in the ablation zone rose to more than 100 °C and lasted for 5 minutes. After the automatic cool down of the radiofrequency system, the tines were retracted and the generator was reactivated to ablate the track from the tumors to the subcutaneous tissues, preventing bleeding or tumor-cell dissemination. The RFA procedure was based on expert consensus on image-guided RFA of pulmonary tumors in China (8).
Post-treatment
All the patients were scanned by CT immediately at 5 min after treatment. Follow-up visits were scheduled at 1-, 3-, 6-, 12- and 24-month after treatment. The following were done at each follow-up visit: physical examination, CT scanning for tumor assessment, recording of concomitant medication and pulmonary function testing. SF-12 and SAS questionnaires were assessed at 12- and 24-month after treatment.
Tumor size, including tumor diameter and tumor volume, and the change of CT value were detected. Tumor volume was compared by using the product of two perpendicular maximum diameters of the tumor. When enhanced CT scanning indicated that abnormal enhancement still could be detected in the center and periphery of lesions, or multiple tumors recrudesced in the ablation zone, tumor residue or local recurrence was considered. All the lesions were assessed by three physicians independently.
Outcome measures
The feasibility assessment was defined as correct placement of the ablation probe into all targeted tumors with completion of the planned ablation protocol.
Effectiveness assessment included complete response (CR) rate, partial response (PR) rate, no change (NC) rate, progressive disease (PD) rate, overall survival and local control rate (CR + PR) in 6-, 12- and 24-month. Effectiveness assessment followed the response evaluation criteria in solid tumors (RECIST) and the expert consensus in China (8,15). CR: tumors disappeared completely, CT scanning could not indicate tumors, no enhancement of the tumors; PR: the product of two perpendicular greatest diameters of tumors minified ≥50%, area of non-enhanced zone ≥50%; NC: the product of two perpendicular greatest diameters of tumors minified <50% or expanded <25%, area of non-enhanced zone <50%; PD: the product of two perpendicular greatest diameters of tumors magnified ≥25%.
Safety assessment included treatment-related complications, major complication rate of RFA and pulmonary function. In the present study, major complication consisted of symptomatic pneumothorax and high fever. Pulmonary function tests including forced expiratory volume (FEV), FEV of predicted, forced vital capacity (FCV) and FCV of predicted.
QoL was assessed by Short Form-12 (SF-12) and Self-Rating Anxiety Scale (SAS) in baseline, 12- and 24-month. The calculation of physical component summary (PCS) and mental component summary (MCS) was based on previous study (16). The rating of SAS score was as follows: total score between 50 and 60 was defined as mild anxiety, total score between 61 and 70 was defined as moderate anxiety, and total score more than 70 was defined as severe anxiety (17).
Statistical analysis
Statistical analyses were performed by SPSS (22.0, USA). Data were presented as mean ± standard deviation (SD) for continuous variables and percentages for dichotomous variables. Differences between baseline value and post-treatment values of tumor size and pulmonary function were tested by use of an unpaired t-test. Overall survival rate and local control rate were estimated by Kaplan-Meier method. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
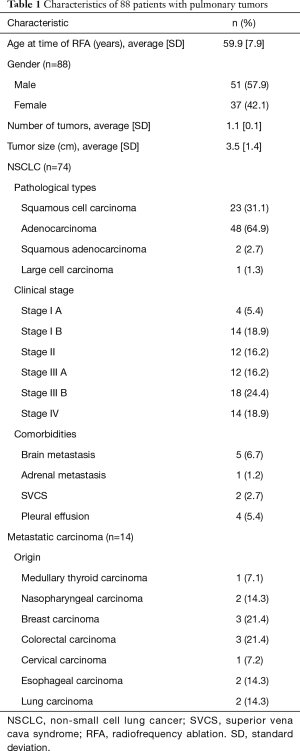
Eighty-eight patients [37 women and 51 men; mean 59.9 years (SD ±7.90)] with 96 lung nodules were collected from January 01, 2012 to December 10, 2016. Average tumor size was 3.5 cm (SD ±1.4). Seventy-four of our patients were diagnosed NSCLC with 76 lung nodules, including 23 squamous cell carcinoma, 48 adenocarcinoma and 2 squamous adenocarcinoma and one large cell carcinoma. All the patients with NSCLC were also classified by clinical stages (4 patients in stage IA, 14 patients in stage IB, 12 patients in stage II, 12 patients in stage IIIA, 18 patients in stag IIIB, 14 patients in stage IV). Twelve of our patients had associated comorbidities before treatments [4 with brain metastases, one with adrenal metastasis, two with superior vena cava syndrome (SVCS) and 4 with pleural effusion] (Table 1). Fourteen of our patients were diagnosed pulmonary metastases. Metastases from medullary thyroid carcinoma in one patient, nasopharyngeal carcinoma in two patients, breast carcinoma in three patients, colorectal carcinoma in three patients, cervical carcinoma in one patient, esophageal carcinoma in two patient, and lung carcinoma in two patients (Table 1).
Full table
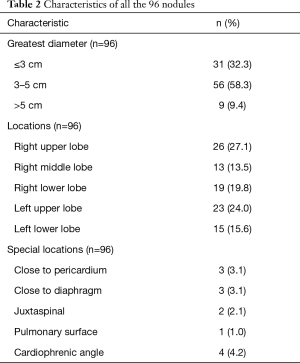
Among all the 96 lung nodules, 31 nodules were not more than 3 cm in greatest diameters, 56 nodules were between 3 cm and 5 cm, and 9 nodules were over 5 cm. 58 nodules were located in right lung (26 in upper lobe, 13 in middle lobe, 19 in lower lobe). And 38 nodules were located in left lung (23 in upper lobes and 15 in lower lobes). 13 nodules occurred in special locations (3 nodules were close to pericardium, 3 nodules were close to diaphragm, 2 nodules were juxtaspinal, 1 nodule was on pulmonary surface and 4 nodules were located in cardiophrenic angle (Table 2).
Full table
Feasibility assessment
Successful placement of the RF ablation probe was achieved in all targeted tumors with completion of the planned ablation protocol (100%). 134 RFA were successfully completed in 88 patients with 96 nodules. Owning to the nonspherical shape, nine of the nodules required repositioning and redeployment to ensure complete ablation. Patients with multiple nodules underwent treatment of only one lesion at each visit. To patients with giant tumors (5–7 cm), area of ablation was from 60% to 90%. Owing to the wide disparity in lesion geometry, surgery time and ablation time varied greatly (surgery time: range, 75–150 min; ablation time: range, 35–115 min, mean, 42.8 min).
Effectiveness assessment
Five minutes after RFA, post-ablation CT was scanned. Shadows expansion and edge blurs were observed in most lesions (92/96, 95.8%). All treated nodules were surrounded by ground-glass opacification, indicating local edema and hemorrhage. Furthermore, low-density zones inside lesions and bubble-like changes could also been observed in some treated lesions.
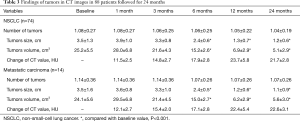
At one month after treatment, the tumor sizes had no significant difference with that in baseline from CT images. However, low-density zone existed in tumor lesions with no vascular enhancement. At 3 months after treatment, tumor shrink and irregular low-density zone were observed in all the treated nodules and some of tumors also displayed cavity-like changes. At 6 months after treatment, further tumor shrink was observed in 88 of 96 nodules (91.7%). The average tumor volume was significantly decreased compared with baseline (15.2±2.6 vs. 25.1±5.5 cm3, P<0.001). Tumor size continued shrinking in the following 18 months (Table 3, Figures 1,2).
Full table
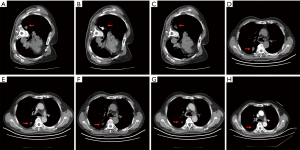
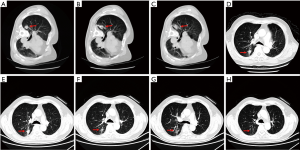
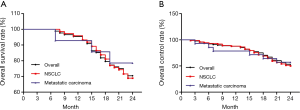
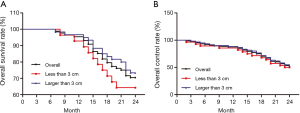
At 6 months, 60.2% patients had CR, 31.9% patients had PR, 4.5% patients had NC and 3.4% patients had PD. The local control rate (CR + PR) was 92.1% and overall survival rate was 100% at 6 months. Four patients died during 6 and 12 months after treatment because of brain metastasis and osseous metastasis. one year overall survival was 95.5%. At 24 months, 36.3% patients had CR, 14.8% patients had PR, 8.0% patients had NC and 11.3% patients had PD. The local control rate was 51.1% and overall survival rate was 70.5 at 24 months (Table 4, Figure 3). There was no difference of survival rate and local control rate between patients with nodes less than 3 cm and those nodes larger than 3 cm (Figure 4).
Full table
Safety assessment
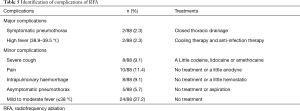
No procedure-related death occurred during or after treatment. During RFA, most of patients felt local pyrexia, perspiration and heart rate acceleration, but there was no necessity for treatment. Pain was complained by 10 patients (11.4%) and intrapulmonary hemorrhage occurred in 8 patients (9.1%) during or after treatment, which all alleviated after expectant treatment. Severe cough occurred in 8 patients (9.1%), which was associated with bronchial irritation and relieved by treating with codeine, lidocaine or oramethocaine. Pneumothorax occurred in 7 patients (7.9%) during or after treatment. Among them, 5 patients (5.7%) were asymptomatic and treated with aspiration and cured after 3–5 days; Two patients (2.3%) were symptomatic and treated by closed thoracic drainage. One patient was treated immediately after surgery and another one was treated at 1 month visit. Twenty-four patients (27.2%) had mild to moderate fever (≤38 °C) and no treatment (NT) needed to be used. High fever (38.9–39.5 °C) occurred in 2 patients (2.3%) and relieved by using cooling therapy and anti-infection therapy. In our study, major complication consisted of symptomatic pneumothorax (2/88) and high fever (2/88) and the major complication rate was 4.5% (4/88). No severe complication occurred during or after treatment, such as bronchopleural fistula (Table 5). Pulmonary function tests did not show significant worsening in FEV, FEV percentage predicted, FCV, and FCV percentage predicted in follow up compared with baseline (Table 6).
Full table

Full table
QoL
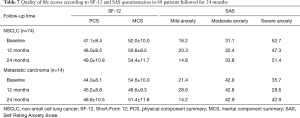
SF-12 physical (PCS) and mental summary scores (MCS) and SAS questionnaire were used to assess the QoL of patients underwent RFA. At baseline, the score of PCS and MCS were 47.1±8.4 and 52.0±10.0 in patients with NSCLC and 44.0±8.1 and 54.6±10.0 in patients with metastatic carcinoma. There was no significant difference in PCS and MCS in the following 12- and 24-month, both in patients with NSCLC and metastatic carcinoma (Table 7). SAS was used to assess the severity of anxiety patients underwent RFA. The proportion of mild, moderate and severe anxiety was 16.2%, 31.1% and 52.7% in patients with NSCLC and 21.4%, 42.9% and 35.7% in patients with metastatic carcinoma at baseline. No significant difference was observed in both NSCLC and metastatic carcinoma at 12- and 24-month (Table 7).
Full table
Discussion
In the present study, we conducted RFA in 96 lung tumor nodes of 88 patients and found good feasibility, effectiveness, safety and QoL. The overall survival rate and local control rate in 6-, 12- and 24-month follow-up were 100%, 95.5%, 70.5% and 92.1%, 87.5% and 51.1%, respectively.
RFA was a recognized technique for treating various kinds of tumor because of its minimally invasive procedure and maximum function reserved (18). Since it was performed in lung tumor in 2000, a series of studies explore the effect of RFA in treating different kinds of lung tumors. In 2008, Lencioni et al. (19) conducted the first prospective, intention-to-treat, multi-center clinical trial with the aim of assessing the feasibility, safety, and effectiveness of RFA in the treatment of lung malignancies. Overall survival was 70% at 1 year and 48% at 2 years in patients with NSCLC. In 2011, Palussiere et al. enrolled 198 consecutive patients (82.8% were metastases and 17.2% were NSCLC) and found the survival rate at 2-, 3-, 5-year was 72%, 60% and 51%, respectively (20). In 2015, Baere et al. (12) reported their experience of RFA in treating lung metastases in 566 patients with 1,037 metastases. They found the 4-year local efficacy was 89% and 4-year lung disease control rate was 44.1%. In the present study, the overall survival rate and local control rate for NSCLC were 95.9% and 87.8% at 1 year and 68.9% and 50.0% at 2 years, which was consistent with previous study. Furthermore, patients with lung metastases were also included and the overall survival rate and local control rate were found to be 78.6% and 57.2% at 2 years.
Tumor size, location, clinical stage and pathological types were all important influence factors for the efficacy of RFA. Lanuti et al. (21) reported a study in 31 patients with small pulmonary tumors and the average diameter of the tumor was 2.0 cm. CR was achieved in all the tumors after RFA and 2- and 4-year overall survival were 78% and 47%. Liu et al. (22) reported a study on 29 medically inoperable patients with clinical stage I NSCLC and the overall survival was 90.5% at 1 year, 76.4% at 2 years, and 65.5% at 3 years. Wang et al. (23) reported a study in 58 stage IV malignant lung tumor patients and the time to local progression, progression-free survival, and overall survival were 15.4±7.5, 9.6±5.8 and 18.0±7.0 months, respectively. Cheng et al. (24) studied on 12 patients with middle stage of NSCLC and found that median overall survival was 35 months and the determine time to local progression was 14 months in tumor >30 mm. In the present study, 31 small pulmonary tumors (greatest diameter ≤3 cm) achieved CR after RFA, and 29 of 31 nodules demonstrated shrink, coagulation, necrosis, even disappearance after 1–12 months with a 92.8% of overall survival rate and 85.7% of local control rate at 1 year. However, it was difficult to place the RFA probe inside the small nodules. On the other hand, irregular low-density zone could be observed in all the treated 65 large nodules (greatest diameter >3 cm) and 59 of 65 nodules shrank in different degree. The overall survival rate and local control rate were 95.0% and 88.3% at 1 year.
Pulmonary function, including early and late postoperative prediction, is vital to patients’ well-being and the choice of procedure. Though surgery resection was effective for patients with favorable histology, it impact on pulmonary function is also obvious. Local treatment has less impact on pulmonary function. Stereotactic body radiation therapy, a local minimally invasive technique, was applied to 292 patients with both early-stage NSCLC and COPD and median values for decline ratio in FEV in 1 second were 7.9%, 7.9%, and 7.4% and median values for decline ratio in FCV were 5.1%, 3.4%, and 0.5% in patients with normal function, mild to moderate and severe COPD, respectively (25). In the present study, another local minimally invasive technique, RFA, was used. No significant changes in pulmonary function (including FEV, FEV of predicted, FCV, FCV of predicted) in a 2-year follow up.
Furthermore, QoL was another factor which took into concern when choosing therapeutic regimen. Huang et al. (26) enrolled 389 patients with small hepatocellular carcinoma who underwent RFA or surgical resection. They found that patients treated with percutaneous RFA had significantly better health-related QoL total scores after 3-, 6-, 12-, 24- and 36-month than those who had surgical resection. Toro et al. (27) compared the QoL in patients with hepatocellular carcinoma who underwent hepatic resection (HR), trans-arterial chemoembolization (TACE), RFA or NT and found HR provides the best QoL at 24 months while RFA provides a worse QoL compared to HR, but a higher QoL compared to TACE or NT. In the present study, no significant worsening of QoL was observed in following 12- and 24-month after treatment, which indicated that RFA was not a bad choice in treating inoperable patients with pulmonary tumor.
Pneumothorax is the primary complication after RFA and the incidence of pneumothorax is 4.5–61.1%. Most of patients can heal by themselves and only some of them (range, 3.3–38.9%; mean, 11%) need closed thoracic drainage (28). Mild pneumothorax usually does not need treatment. Moderate pneumothorax and severe pneumothorax can be treated by aspiration or closed thoracic drainage, which may influence the placement of RFA probe. Repeated placement of RFA probe would make it easier to cause pneumothorax and intrapulmonary hemorrhage (28,29). In the present study, incidence of pneumothorax was 7.9% (7/88). Closed thoracic drainage was used in two patients and others all healed by themselves. Pneumothorax occurred in three patients during the repeated placement of RFA probe, but all the three patients completed RFA after the treatment of aspiration. The keys to avoid pneumothorax are following: skillful operation, accurate localization, avoidance for multiple placement of the RFA probe, avoidance to go through too many lung tissues or two lung lobes, tine of RFA probe should stay inside tumor or lung when RFA probe needs reposition (30).
In conclusion, CT-guided RFA is a feasible, effective, safe therapy and can be an optional treatment for inoperable patients with malignant lung tumors. Prospective, multi-center studies were needed to enhance our understanding on RFA for pulmonary tumors, especially the role of RFA in the treatment combined with radiotherapy, chemotherapy or biological targeted therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was done with the approval of the ethics committee of Nanjing Medical School (No. LLSC2012-08).
References
- Khurana V, Bejjanki HR, Caldito G, et al. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest 2007;131:1282-8. [Crossref] [PubMed]
- Chen WQ, Zhang SW, Zou XN, et al. An analysis of lung cancer mortality in China, 2004 - 2005. Zhonghua Yu Fang Yi Xue Za Zhi 2010;44:378-82. [PubMed]
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26. [Crossref] [PubMed]
- Hiraki T, Gobara H, Iguchi T, et al. Radiofrequency ablation for early-stage nonsmall cell lung cancer. Biomed Res Int 2014;2014:152087. [Crossref] [PubMed]
- de Baere T, Tselikas L, Gravel G, et al. Lung ablation: Best practice/results/response assessment/role alongside other ablative therapies. Clin Radiol 2017;72:657-64. [Crossref] [PubMed]
- Chen H, Senan S, Nossent EJ, et al. Treatment-Related Toxicity in Patients With Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;98:622-31. [Crossref] [PubMed]
- Thompson SM, Schmitz JJ, Schmit GD, et al. Image-Guided Thermal Ablative Therapies in the Treatment of Sarcoma. Curr Treat Options Oncol 2017;18:25. [Crossref] [PubMed]
- Liu BD, Zhi XY. Expert consensus on image-guided radiofrequency ablation of pulmonary tumors-2015 edition. Ann Transl Med 2015;3:128. [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [Crossref] [PubMed]
- Tavares ECA, Freitas S, Portilha A, et al. Efficacy and safety of percutaneous radiofrequency thermal ablation in the treatment of lung cancer lesions. Acta Med Port 2015;28:63-9. [Crossref] [PubMed]
- Modesto A, Giron J, Massabeau C, et al. Radiofrequency ablation for non-small-cell lung cancer in a single-lung patient: case report and review of the literature. Lung Cancer 2013;80:341-3. [Crossref] [PubMed]
- de Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987-91. [Crossref] [PubMed]
- Safi S, Rauch G. Sublobar Resection, Radiofrequency Ablation or Radiotherapy in Stage I Non-Small Cell Lung Cancer. Respiration 2015;89:550-7. [Crossref] [PubMed]
- Singh N, Agarwal R, Aggarwal AN. Quality-of-life assessment in trials of lung cancer. Lancet 2007;370:933-author reply 933-4. [Crossref] [PubMed]
- Ding Q, Cheng X, Yang L, et al. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J Thorac Dis 2014;6:677-83. [PubMed]
- Lam CL, Tse EY, Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual Life Res 2005;14:539-47. [Crossref] [PubMed]
- Delibegovic A, Sinanovic O. The influence of palliative care on the level of anxiety and depression in lung cancer patients. Med Arch 2013;67:263-5. [Crossref] [PubMed]
- Akhan O, Guler E, Akinci D, et al. Radiofrequency ablation for lung tumors: outcomes, effects on survival, and prognostic factors. Diagn Interv Radiol 2016;22:65-71. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Palussiere J, Marcet B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011;34:989-97. [Crossref] [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [Crossref] [PubMed]
- Liu B, Liu L, Hu M, et al. Percutaneous radiofrequency ablation for medically inoperable patients with clinical stage I non-small cell lung cancer. Thorac Cancer 2015;6:327-33. [Crossref] [PubMed]
- Wang Y, Li G, Li W, et al. Radiofrequency ablation of advanced lung tumors: imaging features, local control, and follow-up protocol. Int J Clin Exp Med 2015;8:18137-43. [PubMed]
- Cheng M, Fay M, Steinke K. Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: A retrospective study. Int J Hyperthermia 2016;32:316-23. [Crossref] [PubMed]
- Takeda A, Enomoto T, Sanuki N, et al. Reassessment of declines in pulmonary function >/=1 year after stereotactic body radiotherapy. Chest 2013;143:130-7. [Crossref] [PubMed]
- Huang G, Chen X, Lau WY, et al. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg 2014;101:1006-15. [Crossref] [PubMed]
- Toro A, Pulvirenti E, Palermo F, et al. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol 2012;21:e23-30. [Crossref] [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [Crossref] [PubMed]
- Tongdee T, Tantigate P, Tongdee R. Radiofrequency Ablation of Lung Metastasis Not Suitable for Surgery: Experience in Siriraj Hospital. J Med Assoc Thai 2015;98:1019-27. [PubMed]
- Hu M, Zhi X, Zhang J. Radiofrequency ablation (RFA) for palliative treatment of painful non-small cell lung cancer (NSCLC) rib metastasis: Experience in 12 patients. Thorac Cancer 2015;6:761-4. [Crossref] [PubMed]


