Effect of chest tube size on pleurodesis efficacy in malignant pleural effusion: a meta-analysis of randomized controlled trials
Introduction
Malignant pleural effusion (MPE) develops in approximately 50% of all patients with metastatic cancer (1,2). It is caused by direct pleural invasion of tumor cells resulting in increased permeability of the pleural microvessels and involvement of local lymph nodes, causing reduced fluid reabsorption (2-5). The estimated incidence of MPE is about 175,000 in the United States each year, according to data collected more than a decade ago (6). This incidence is expected to be even higher presently as the global burden of malignancy continues to increase. In addition, MPE is believed to be present in up to 15% of patients who die with malignancies (4).
The average life expectancy varies by malignancy type and performance status of patients but is expected to be between 3 to 9 months (7). MPE causes increased shortness of breath and significant reduction in quality of life requiring multiple hospital admissions.
Although thoracentesis can provide rapid relief of symptoms, it does not prevent the recurrence of effusion and eventually symptoms. Therefore, definitive control of recurrent MPE is needed to achieve better quality of life and maximize out of hospital stay. Currently, this could be achieved by intrapleural sclerosing agents through a chest tube or indwelling pleural catheters to achieve pleurodesis.
Pleurodesis is the procedure aiming at adhere the visceral and parietal pleura, which causes an obliteration of the pleural space. The optimal strategy for pleurodesis regarding the size of chest drain remains unsettled (3,7).
Pleurodesis and chest tube insertion are painful (8). The optimal chest tube size for pleurodesis has not yet been identified, though numerous clinical studies have been performed to try to determine the optimal chest tube. The British Thoracic Society guideline (3) advocates and recommends the use of smaller tubes over large tubes for drainage and pleurodesis in patients with MPE. Small-bore tubes require a smaller incision and less tissue dissection. Small-bore tubes were also found to cause significantly less pain both during insertion and once in place compared with larger-bore tubes in patients with pleural infection (9). However, smaller tubes may be less safe, with one observational study reporting a higher proportion of complications (10).
In clinical practice, it appears that there is still no consensus on the optimal size of chest tube in patients with MPE (11,12). We performed this meta-analysis based on published randomized controlled trials (RCTs) to evaluate the overall efficacy and safety of small chest tube compared with large chest tube in patients with MPE.
Methods
Literature search
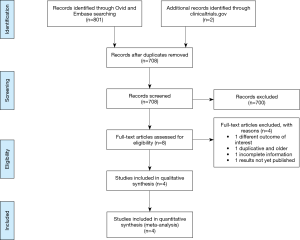
We searched Medline (Ovid) and EMBASE records (from 1980 to March 2016) using a predefined algorithm strategy (Figure 1). We used “pleural effusion” and “malignancy” and their synonyms as keywords. We restricted the search to RCTs in humans, without any language restrictions.
Study eligibility
We included studies that assessed the efficacy of pleurodesis in MPE patients treated with small vs. large chest tubes inclusion and exclusion criteria were determined a priori. We included only RCTs. Retrospective studies, case studies and review articles were excluded. Studies recruiting both malignant and non-malignant participants with no clear distinction between the two groups in the results section and studies that did not include data regarding the efficacy of pleurodesis were also excluded.
For this study, successful pleurodesis was defined as no need for a repeat pleural intervention to manage symptomatic effusion after chest tube removal (as per current clinical practice). The chest tube sizes were divided based on measurement of French (F): ≤14 F is considered small bore and >14 F is considered large bore. Types of drugs used intrapleurally to induce pleurodesis were ignored if they had a similar effect.
All the references identified were imported into the bibliographic database Endnote and duplicates were removed. The titles and abstracts retrieved by the search were screened for relevance independently by two authors. Potentially eligible studies were identified and we obtained the full papers, which were then independently assessed for inclusion by at least two authors. Any disagreements, at both steps, were resolved through discussion among all the authors. This process was facilitated by use of the Rayyan systematic reviews web application (http://rayyan.qcri.org/), which is compatible with Endnote. All data extracted were recorded on a standardized data abstraction form.
Data extraction
We recorded information on study characteristics and demographics including authors, publication year, journal, sample size, demographics, cancer type, the intervention details, the duration of follow-up, primary outcome (success proportion), adverse events and pain symptoms from chest tube size as well as information regarding randomization mode, allocation concealment, blinding, loss to follow-up, intention-to-treat analysis and selective reporting. Data extraction was performed independently by at least two investigators for each study and disagreements were resolved by consensus.
Quality assessment
Quality assessment of the included studies was done by all reviewers using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (13). The domains used in the present systematic review pertain to randomization and allocation concealment (selection bias), blinding (performance and detection bias) and loss to follow-up and adherence to the intention-to-treat principle (attrition bias) as well as selective reporting. Among the established strategies, we chose to present the meta-analysis of all studies while providing a summary of the risk of bias across studies. For all studies, participants and personnel could not be blinded to which intervention a participant received, as the size of the chest tube is readily apparent.
Statistical analysis
The raw data on numbers of total participants and events was retrieved from included studies and a correction factor of 0.5 was added to case counts for studies with zero events. Overall relative risk (RR) with 95% confidence intervals (CI) was pooled using random-effects model. The presence of statistically significant heterogeneity was assessed by the Q statistic (significant at P<0.10) and the extent of the observed heterogeneity was assessed by the I2 (ranging from 0% to 100%, with a value greater than 50% was recognized as indicative of substantial heterogeneity as determined a priori) (14). We expected a degree of clinical heterogeneity between the included study results because of the different countries in which the studies were based, sclerosing agents used, methods used to define pleurodesis and the different time points that was assessed at. We therefore used the random-effects model for pooling RR and 95% CI. To detect publication bias, we visually examined funnel plots and further assessed asymmetry by using the Begg and Egger tests (15). In addition; the trim-and-fill approach was used to obtain an adjusted effect size that takes into account publication bias. Furthermore, we performed sensitivity analyses using influential meta-analysis to assess the effect of individual study on overall RR. All P values are 2-tailed and a P<0.05 was considered statistically significant. All analyses were performed using STATA software, version 12.0 (STATA Corp., College Station, TX, USA).
Results
Literature search
An electronic database search retrieved 801 citations. After duplicates were removed, abstracts of 708 citations were reviewed and 8 potential studies to include were identified, for which full text articles were obtained. All abstracts were available in English, regardless of the study country and language of the article; of the eight full-text articles reviewed, seven were in English and one was in Chinese, all of which were accessible for investigators on the team. After examining those articles in more detail, four articles were excluded for reasons shown in Figure 1 (16-23). Four studies met the inclusion criteria and were considered in this review (16-19).
Study characteristics
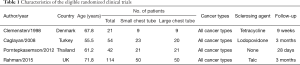
Summary and study characteristics are shown in Table 1. The four included studies were from four different countries (Denmark, Turkey, Thailand and UK) and comprised 231 participants, with 103 participants allocated to small tube group and 100 participants to large tube group. Twenty eight participants were lost to follow-up because of death or unavailability to assess outcome (16-19). The average age of participants ranged from 55.5 to 71.8 years, and the average length of follow-up after treatment ranged from 28 days to 3 months. Other demographic information was not consistently available within and across studies. Different sclerosing agents, including talc, tetracycline and povidone iodine, were used in conjunction with the chest tubes.
Full table
Risk of bias in included studies
The studies were of high methodological quality in most areas of potential bias as defined in the Cochrane Handbook (13). Regarding allocation concealment and blinding, it is not possible to blind investigators and patients regarding chest tube size. Otherwise, studies were of high methodological quality. A clearer breakdown of the quality of the studies is described in Table 2.

Full table
Overall efficacy
All four studies were included for assessing pleurodesis efficacy of small vs. large chest tube. Three studies (17-19) showed that large tube had a trend toward better efficacy than small tube but without statistical significance (Table 3). The pooled estimation showed that there was no statistically significant difference when comparing small with large tubes, with a pooled RR of 0.90 (95% CI, 0.77–1.05; P=0.19; I2 =17.4%; Figure 2). Successful pleurodesis proportion for small versus large chest tubes was 73.8% vs. 82.0%. Sensitivity analysis excluding the oldest study (17), which had the smallest sample size and unclear randomization information, gave similar results (Figure 2).

Full table
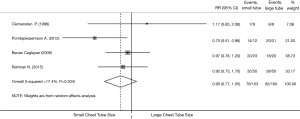
Complication proportion
All four studies were included for assessing the complications of small and large chest tubes (Table 3) (16-19). The pooled estimation showed that there was no statistically significant difference when comparing small with large tubes, with a pooled RR of 0.95 (95% CI, 0.42–2.15; P=0.90; I2 =0.9%; Figure 3). Complication proportion for small vs. large chest tubes was 13.0% vs. 10.5%. Sensitivity analysis excluding the oldest (16), which had the smallest sample size and unclear randomization information, gave similar results (Figure 3). We attempted to compare pain while tube was in situ between small vs. large bore chest tubes. However, different methods were employed to measure pain between groups and it was not possible to meta-analyze such an outcome. Two studies (18,19) used two different pain scales, one study (16) used a narrative assessment of pain, and another study (17) used a binary assessment of pain.

Publication bias
Since there are only four studies included in this meta-analysis, it is not adequate to assess for publication bias.
Discussion
The results of the current meta-analysis suggest that the use of small-bore tube is as effective as large-bore tube for successful pleurodesis in patients with MPE. In addition, the complication proportion was similar between the two groups.
In this current analysis, we also attempted to compare pain between large and small chest tube techniques. However, studies assessed pain differently. Porntepkasemson et al. used a pain scale (from 0 to 10) which did not differ significantly among both groups (18). Rahman et al. used a visual analog scale (VAS) from 0 to 100 mm (19). Although larger tubes were associated with statistically significantly more pain (6 mm adjusted difference), it did not reach clinical significance (the minimum clinically significant threshold for a 100 mm VAS pain score is 13 mm). Another study (16) only described that patients with large tube found it unpleasant compared to small catheter without objectively assessing pain and Caglayan et al. mentioned that 15% of patients with large catheter had pain compared to 17.3% in patients with small catheter (17).
The largest systematic review and meta-analysis (25) of MPE published in 2004 posed key questions that are as yet unanswered. One of the questions was whether “the use of small bore catheters for pleurodesis is as effective as large bore catheters.” Furthermore, the current British Thoracic Society guideline (3) advocated the use of small-bore tubes as the initial choice for effusion drainage and pleurodesis. This was based on (1) two randomized studies (16,17) and one retrospective trial (25) which showed equal pleurodesis efficacy and (2) non-comparative studies using small-bore tubes with sclerosants that reported similar success proportions to large-bore tubes and appeared to cause less discomfort (26-29). However, two other randomized studies comparing small and large bore chest tube for efficacy, complications and objective pain measures have been published in the literature (18,19). In this meta-analysis, we attempted to answer the question about the effect of chest tube size on pleurodesis based on recently published RCTs.
On the basis of this current meta-analysis, what should now be the recommended size of chest tube for pleurodesis? Until further evidence emerges, the use of small and large-bore chest tubes for MPE pleurodesis is equally efficacious. While it was not possible to pool pain data across studies, larger tubes were not associated with clinically significant more pain than smaller tubes when objective pain scores were used (18,19). These data highlight and emphasize the need for adequately powered studies addressing specific clinical management issues in common pleural diseases such as MPE along with documenting quality of life using standardized measures.
It is important to emphasize that the goal of treatment for patients with MPE and limited life expectancy is to alleviate symptoms effectively, prevent recurrence of effusion and minimize pleural interventions without causing further pain. When making a choice in clinical practice between different chest tube sizes or whether sclerosing agent should be used, clinicians should also take into account the economic cost, patients’ performance status as well as their preference.
The strength of this study is that, while it includes studies from multiple countries, the search and inclusion was not limited by language. Furthermore, it questions previous guidelines (3) that the use of small chest tube over large chest tube is preferred.
However, there were also limitations, including the small number of studies meeting the criteria. While study quality was generally high, participants and investigators could not be blinded to intervention in any of the trials, which could have led to performance bias. Also, most studies were small which might affected the estimate of effect size. Publication bias is a significant threat to the validity of the results, and we could not adequately assess such in the present meta-analysis due to the limited studies found. Further studies comparing small and large bore chest tubes for pleurodesis success along with complication proportion and objective pain score measurement, such as VAS, while chest tube is in situ, need to be performed.
In conclusion, this meta-analysis of four prospective RCTs comparing efficacy and safety of small vs. large bore chest tube size suggested that both modalities are effective in achieving pleurodesis with similar complication proportion and comfort level.
Acknowledgements
The authors thank Dr. Tanika Kelly for her guidance and assistance through this process.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spiegler PA, Hurewitz AN, Groth ML. Rapid pleurodesis for malignant pleural effusions. Chest 2003;123:1895-8. [Crossref] [PubMed]
- Rodrîguez-Panadero F, Borderas Naranjo F, López Mejîas J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989;2:366-9. [PubMed]
- Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. [Crossref] [PubMed]
- Sears D, Hajdu SI. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol 1987;31:85-97. [PubMed]
- DiBonito L, Falconieri G, Colautti I, et al. The positive pleural effusion. A retrospective study of cytopathologic diagnoses with autopsy confirmation. Acta Cytol 1992;36:329-32. [PubMed]
- Marel M, Zrůstová M, Stasný B, et al. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest 1993;104:1486-9. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest 2003;124:2229-38. [Crossref] [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [Crossref] [PubMed]
- Collop NA, Kim S, Sahn SA. Analysis of tube thoracostomy performed by pulmonologists at a teaching hospital. Chest 1997;112:709-13. [Crossref] [PubMed]
- Lee HJ, Feller-Kopman DJ. POINT: Should Small-Bore Pleural Catheter Placement Be the Preferred Initial Management for Malignant Pleural Effusions? Yes. Chest 2015;148:9-10. [Crossref] [PubMed]
- Gillespie CT, DeCamp MM. COUNTERPOINT: Should Small-Bore Pleural Catheter Placement Be the Preferred Initial Management for Malignant Pleural Effusions? No. Chest 2015;148:11-3. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193-206. [Crossref] [PubMed]
- Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993;2:121-45. [Crossref] [PubMed]
- Clementsen P, Evald T, Grode G, et al. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92:593-6. [Crossref] [PubMed]
- Caglayan B, Torun E, Turan D, et al. Efficacy of iodopovidone pleurodesis and comparison of small-bore catheter versus large-bore chest tube. Ann Surg Oncol 2008;15:2594-9. [Crossref] [PubMed]
- Porntepkasemson A, Geater SL. Comparison of pain and efficacy between large-bore and small-bore chest tube drainage for malignant pleural effusion in Songklanagarind Hospital: A randomized controlled trial. J Clin Oncol 2012;30:e19608.
- Rahman NM, Pepperell J, Rehal S, et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Bhatnagar R, Laskawiec-Szkonter M, Piotrowska HE, et al. Evaluating the efficacy of thoracoscopy and talc poudrage versus pleurodesis using talc slurry (TAPPS trial): protocol of an open-label randomised controlled trial. BMJ Open 2014;4:e007045. [Crossref] [PubMed]
- Gou H, Hou M, Zhu J, et al. Zhongguo Fei Ai Za Zhi 2005;8:459-61. [A randomized study of small bore catheter thoracostomy closed drainage versus conventional pleural aspiration in the treatment of malignant pleural effusions]. [PubMed]
- Sahin U, Unlü M, Akkaya A, et al. The value of small-bore catheter thoracostomy in the treatment of malignant pleural effusions. Respiration 2001;68:501-5. [Crossref] [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Primary Result of the 1st Therapeutic Interventions in Malignant Effusion (TIME1) Trial: A 2x2 Factorial, Randomised Trial of Chest Tube Size and Analgesic Strategy for Pleurodesis in Malignant Pleural Effusion. Thorax 2015;70:A15-6. [Crossref]
- Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004.CD002916. [PubMed]
- Parulekar W, Di Primio G, Matzinger F, et al. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest 2001;120:19-25. [Crossref] [PubMed]
- Seaton KG, Patz EF Jr, Goodman PC. Palliative treatment of malignant pleural effusions: value of small-bore catheter thoracostomy and doxycycline sclerotherapy. AJR Am J Roentgenol 1995;164:589-91. [Crossref] [PubMed]
- Morrison MC, Mueller PR, Lee MJ, et al. Sclerotherapy of malignant pleural effusion through sonographically placed small-bore catheters. AJR Am J Roentgenol 1992;158:41-3. [Crossref] [PubMed]
- Parker LA, Charnock GC, Delany DJ. Small bore catheter drainage and sclerotherapy for malignant pleural effusions. Cancer 1989;64:1218-21. [Crossref] [PubMed]
- Patz EF Jr, McAdams HP, Erasmus JJ, et al. Sclerotherapy for malignant pleural effusions: a prospective randomized trial of bleomycin vs doxycycline with small-bore catheter drainage. Chest 1998;113:1305-11. [Crossref] [PubMed]