Cost-effectiveness analysis of sealant impact in management of moderate intraoperative alveolar air leaks during video-assisted thoracoscopic surgery lobectomy: a multicentre randomised controlled trial
Introduction
Despite the adoption of surgical techniques such as “fissure-last lobectomy” and the use of stapling devices to divide fissures, intraoperative alveolar air leak (IOAAL) is one of the most common complications after video-assisted thoracoscopic surgery (VATS) lobectomies. Although many air leaks resolve spontaneously within 48 hours, some persist for days. Patients with postoperative air leaks (PALs) have longer drainage time and a higher incidence of postoperative complications (1-3); therefore, PAL extended the length of stay (LOS) and increased costs. Several risk factors of PAL have been identified, including age, body mass index (BMI), surgeon experience, surgical site (upper lobectomies and bilobectomy), reduced pulmonary function, and pleural adhesions (4-6). The best management of IOAAL is done during surgery. The adopted techniques are over-sewing or stapling areas of air leakage and using sealing agents to treat leaks (7). Nevertheless, during VATS, stitching is very troublesome and time-consuming, whereas using a spray sealant is more comfortable and faster. It is well known that its use increases the costs. In literature, it is not well defined which amount of IOAAL would be self-limiting without other treatments. Therefore, the indication to use a lung sealant is not clear. We conducted a prospective multicentre randomised controlled trial to demonstrate if the use of a polymeric biodegradable sealant (ProgelTM Pleural Air Leak Sealant, Bard Davol, Warwick, RI, USA) reduces PAL in moderate IOAAL compared with standard treatments and with the aim of a cost-effectiveness analysis.
Methods
A multicentre randomised controlled, parallel group, study with balanced allocation ratio 1:1 was designed. This study was spontaneous, independent, and without direct or indirect profit. The customer was the Italian VATS group (www.vatsgroup.org). The study was conducted according to the ethical principles of the Declaration of Helsinki and by Good Clinical Practice. The schedule for inclusion was determined by the patient’s admission to hospital. All patients were required to provide written approved consent before enrolment. The study protocol was first approved by the local ethics committee of the coordinating centre of Bolzano (N. 1-2014 bis, 30/07/2014) and, then, from local board of each participating centre. The trial population comprises patients older than 18 years scheduled for elective lobectomy or bilobectomy performed by VATS for proven or suspected primary lung cancer or centrally located pulmonary metastasis of extrapulmonary malignancies. The completeness of fissures was evaluated according to the Craig and Walker’s classification (8): grade 1—complete fissure with entirely separate lobes; grade 2—complete visceral cleft but parenchymal fusion at the base of the fissure; grade 3—visceral cleft evident for part of the fissure; grade 4—complete fusion of the lobes with no visible fissures line. At the end of VATS lobectomy, the air leak was demonstrated by saline submersion test and a standardized Ventilation Mechanical Test (VMT): duration 1 minute of volumetric ventilation with the constant flow with a peak pressure of 22 cmH2O, 12 respiratory rates per minute, and a positive end-expiratory pressure (PEEP) of 5 cmH2O. A previous check was made after double-lumen intubation, before surgery, to exclude leak due to ventilator system or endotracheal tube. In the presence of IOAAL, leakage was graded according to a VMT as mild (leak <100 mL/min), moderate (leak =100–400 mL/min) and severe (leak >400 mL/min). Patients with severe leak underwent further treatment using standard procedures including parenchymal stapling or suturing followed by another VMT until the leak was downgraded. Patients who downgraded to moderate IOAAL after treatment were enrolled in the study. Patients with mild leakage were excluded from the study because self-limiting in most cases. Patients presenting moderate air leakages at the end of VATS lobectomies constituted the study population and were randomised to Sealant Group or Control group. The randomisation sequence was created using PHP, Apache and MySQL with a 1:1 allocation using two random block sizes and was available through personal identification. Patients randomised to the Sealant group were treated with the application of the sealant to each of the identified air leaks. If an air leak was still present, surgeons could reapply sealant only up to two more times. After the application, ventilation to the treated lung was suspended for 2 minutes and a second VMT was then repeated and recorded.
The device utilised is a spray, albumin-based hydrogel sealant.
Patients randomly allocated Control Group B received no treatment. At the end of the operation, a 24 Fr coaxial smart drain tube (Round Coaxial Drain; Redax®, Mirandola, Italy) was inserted through the camera port with the tip at the apex of the chest cavity, regardless of the type of lobectomy performed. In all patients, immediately after surgery, the chest tube was collected to an active suction system with a continuous negative pressure of 20 cmH2O for 24 h, after which they were placed to water seal. Postoperatively, PAL volume (mL/min) was measured using a digital mass airflow sensor device (DrentechTM Palmevo, Redax®, Mirandola, Italy) connected to the chest drainage suction unit with portable vacuum unit and rechargeable battery. The real-time PAL data were digitally recorded postoperatively, stored for 100 h and downloaded through a mini USB key port. We recorded the mean 24-h PAL value. The drainage was removed when there was no residual air leak following the switch to water seal, the lung had expanded sufficiently, and amount of drained fluid <250 mL/day. The PAL duration was measured from the day of surgery until the chest tube was removed. Chest roentgenograms were obtained after surgery, within 24 h before and after chest tube removal, and at 1 and 2-month follow-up.
The primary efficacy endpoint of the study was the reduction of PAL duration. The secondary outcomes measures included: mean time to chest drain removal, mean LOS, the percentage of postoperative complications occurring within 2 months, and cost analysis. Complications were considered as a composite rate of the device- and procedure-related events. For the economic evaluation, the duration of surgery, the price of the sealant, and cost of 1 day of hospital stay were evaluated. The average price of the Sealant (ProgelTM) was 445€. Mean hospitalisation cost per day was 750€.
Statistical analysis
A CONSORT checklist was completed (9). A power analysis was performed to calculate the sample size for unpaired analysis. To detect a reduction in PAL of 2 days between the two groups, according to the Cochrane Database Review (10) and Klijian et al. (11), a sample size of at least 48 procedures was needed, with a type 1 error rate set at 0.05 and power at 0.80. To recruit this number of patients a 24-month inclusion period was anticipated. Continuous variables are presented as mean ± standard deviation. The two operational setups were compared using Student’s t-test or Wilcoxon’s two-sample test (discrete or continuous data) and Pearson’s χ2 test or Fisher’s exact test when appropriate (dichotomous or categorical data). The significance level was set at 0.05 for all parameters. The software Stata 9.0 (Stata Corp, College Station, TX, USA) was used for the statistical analysis.
Results
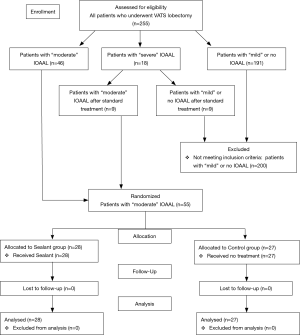
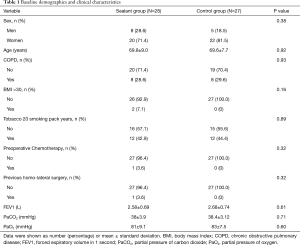
Between January 2015 and January 2017, written informed consent was obtained from 255 patients, who underwent VATS lobectomy; 55 with moderate air leak during the underwater air-tightness test were randomly assigned to the two groups (Sealant group =28 patients, Control group =27 patients). All participants were followed-up at 1 and 2 months. The flow of participants through each arm of the trial was depicted in Figure 1. The baseline demographic, surgical, and pathological characteristics of randomised patients in the two study groups are shown in Tables 1 and 2. The two arms were well balanced, and there were no statistically significant differences between them. The indication for pulmonary resection was primary lung cancer in 66.7% with an equal distribution in the two patient groups. All patients received VATS procedure. After VATS lobectomy, 46 (18.0%) patients had moderate IOAAL. Ninty-six point four percent [27/28] of patients in the Sealant Group A were treated with one application of Progel; in 1 patient (3.6%) claim was repeated once. Eighteen patients (7.1%) with severe IOAAL underwent standard treatments. Nine patients downgraded to moderate IOAAL and were enrolled in the study. Nine patients who downgraded to mild or no leaks were not enrolled.
Full table
Full table
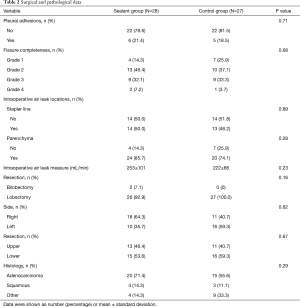
In the Control group, no further intervention was performed; no sealant application was used. There were also no differences in the postoperative management of patients in both groups. The location (parenchyma versus stapled lines) and the quantity of IOAAL in both groups were not significantly different (Table 2). There were no statistically significant differences regarding the other characteristics, including intraoperative risk factors for IOAAL (Table 2). There were no device-related adverse events. Prolonged PAL (>5 days) incidence was significantly higher in control group (P=0.018). The in-hospital mortality and 30-day mortality for both groups were absent.
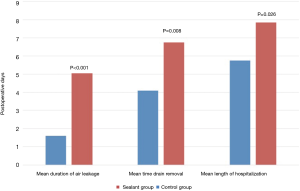
Table 3 showed the outcomes data. The mean PAL duration was statistically different between the two groups (Sealant group =1.60 days versus Control group =5.04 days, P<0.001) (Figure 2). The average duration of chest drainage was statistically different in the two groups (Sealant group =4.1 days versus Control group =6.74 days, P=0.008). The mean LOS was also statistically shorter sealant group (Sealant group =5.75 days versus Control group =7.85 days, P=0.026).

Full table
There was no significant difference in the mean operation time between groups (Sealant group =142±37 minutes versus Control group =151±50 minutes, P=0.49), despite sealant application. Costs due to medical devices were revealed only in the group A (the average price of Sealant per application was 445€). On the other hand, mean hospital stay was 2.1 days shorter in the Sealant group. We compared additional costs in the two groups. In group A cost of the sealant was 12,905€; in group B cost of the longer additional stay was 39,690€ (P<0.001) (Table 4). At 2 months follow-up, no patients required reoperation or chest drainage placements in both groups.

Full table
Discussion
The clinical and economic impact of IOAAL after major pulmonary resection is well known and significant (1-3). Their reduction decreases LOS, postoperative complications and related costs. The best management of IOAAL is done during surgery, but standard techniques are usually inadequate, troublesome and time-consuming during VATS lobectomies (10). A Cochrane Database Review demonstrated that surgical sealants after open pulmonary resection reduce PAL and time to chest drain removal, but failed to show a reduction in LOS (10). Also, an exact measurement of the IOAAL was not performed and represented a potential bias. Many PALs will resolve spontaneously within 48 hours; some will persist. According to data from the Italian VATS group registry, the incidence of PAL (>7 days) is 7.99%. Therefore, a correct intraoperative evaluation of IOAAL is critical and challenging to achieve. Authors adopted the visual grading of air leaks according to Macchiarini scale (0—no leakage; 1—mild, countable bubbles; 2—moderate, stream of bubbles; 3—severe, coalescent bubbles) (12). In our study, a quantitative grading of IOAAL was obtained by a standardised VMT, and this precise and objective classification represents one of strength. The study was focused on patients with moderate IOAAL where the need for intraoperative lung sealant is still controversial. To achieve intraoperative air leak sealing, a spray sealant, in our opinion, was the best solution. We did not observe a significant difference of operation time in the two groups. Sealant polymerises to form a transparent, flexible hydrogel matrix that adheres to the lung tissue within 15 seconds and forms a flexible seal that can withstand 30 mmHg air pressures within 2 minutes of application and a maximum burst pressure of greater than 90 mmHg in less than 10 min. The material is wholly reabsorbed within 1 month postoperatively, and no complications were observed. The thoracic drainage system (air leak data digitally recorded) utilised from each centre allowed a precise analyse at the time of PAL cessation. To the best of our knowledge, this is the first report of a randomised controlled trial investigating the clinical and economic outcomes of a sealant in IOAAL following VATS lobectomy. A recent study examined the use of same biodegradable spray polymer for the closure of IOAAL after minimally invasive pulmonary resection was not a randomised controlled trial (13). Other randomised controlled trials analysing lung sealant efficacy were almost exclusively in patients undergoing thoracotomy (14-16). Cost analysis showed a significant reduction of hospitalisation costs, although no significant differences in postoperative complications were showed. The costs of 2 days of hospitalisation plentifully exceed the price of the lung sealant.
There are several limitations of this study. First, because the trial involved only experienced surgeons in four centres, the results cannot be generalised to other clinical settings. Second, only VATS lobectomies were observed. Nonetheless, since VATS is finding an ever-increasing role in the diagnosis and treatment of a wide range of thoracic disorders, larger-scale studies are desirable to better understand the role of sealant in other VATS procedures like sleeve lobectomy and anatomical segmentectomy. The moderate air leak was defined between 100 and 400 mL/min based on experience. Since data of patients with mild air leaks were not prospectively recorded, we could not demonstrate that they are self-limiting. Furthermore, investigators were not blinded to randomisation, which could influence the time of chest tube removal and patient discharge. Nevertheless, we tried to reduce this bias by following the strict indications reported in the study protocol of postoperative chest tube removal (see Methods).
Conclusions
This randomised controlled trial demonstrated that the use of a Sealant in moderate IOAAL (100–400 mL/min) after VATS lobectomy is safe and efficient. Compared with the control group, Sealant significantly reduced the PAL, the time to drain removal and the LOS and resulted in significant cost saving benefits.
Acknowledgements
We thank Dr. Andrea Ponzoni for his assistance to the biostatistical elaboration.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted according to the ethical principles of the Declaration of Helsinki and by Good Clinical Practice. The schedule for inclusion was determined by the patient’s admission to hospital. All patients were required to provide written approved consent before enrolment. The study protocol was first approved by the local ethics committee of the coordinating centre of Bolzano (N. 1-2014 bis, 30/07/2014) and, then, from local board of each participating centre.
References
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10; discussion 1210. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Stolz AJ, Schützner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. [Crossref] [PubMed]
- Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery. J Thorac Dis 2017;9:1219-25. [Crossref] [PubMed]
- Merritt RE, Singhal S, Shrager JB. Evidence-based suggestions for management of air leaks. Thorac Surg Clin 2010;20:435-48. [Crossref] [PubMed]
- Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4. [PubMed]
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for parallel reporting group randomised trials. Ann Intern Med 2010;152:726-32. [Crossref] [PubMed]
- Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010.CD003051. [PubMed]
- Klijian A. A novel approach to control air leaks in complex lung surgery: a retrospective review. J Cardiothorac Surg 2012;7:49. [Crossref] [PubMed]
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8. [Crossref] [PubMed]
- Park BJ, Snider JM, Bates NR, et al. Prospective evaluation of biodegradable polymeric sealant for intraoperative air leaks. J Cardiothorac Surg 2016;11:168. [Crossref] [PubMed]
- D’Andrilli A, Andreetti C, Ibrahim M, et al. A prospective randomized study to assess the efficacy of a surgical sealant to treat air leaks in lung surgery. Eur J Cardiothorac Surg 2009;35:817-20; discussion 820-1. [Crossref] [PubMed]
- Lequaglie C, Giudice G, Marasco R, et al. Use of a sealant to prevent prolonged air leaks after lung resection: a prospective randomized study. J Cardiothorac Surg 2012;7:106. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]


