Predictors of ventricular tachyarrhythmia occurring late after intracardiac repair of tetralogy of Fallot: combination of QRS duration change rate and tricuspid regurgitation pressure gradient
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, and its surgical outcomes had been dramatically improved due to modern surgical and medical advances. The majority of post-repaired patients survive and reach adulthood, although some patients are at risk of several life-threatening complications, such as arrhythmia and sudden cardiac death (SCD) (1,2). Numerous reports have identified risk factors for ventricular tachyarrhythmia, including QRS duration, change rate of QRS duration, QTc duration, delay in corrective surgery, number of previous cardiac surgery, presence of ventriculotomy, trans-annular patch (TAP) repair, and left ventricular diastolic dysfunction (3-7). Those risk factors probably differ between institutions and countries, since the age at corrective surgery after palliative operation and operative procedure (incidence of TAP repair and size of the right ventriculotomy) might differ between them. The purpose of this study was to evaluate the long-term outcomes of total repair of TOF and to identify risk factors for ventricular tachyarrhythmia and SCD.
Methods
Patients
Our institutional review board/ethical committee approved this retrospective study, and the need for informed consent was waived. Between 1964 and 2014, 415 patients underwent total repair for TOF at Niigata University Medical and Dental Hospital. Cases associated with pulmonary atresia, atrioventricular septal defect; absent pulmonary valve syndrome and major aortopulmonary collateral artery were excluded. Of the 415 patients, we retrospectively reviewed case histories of 89 patients who underwent repair of TOF and who were followed for more than 10 years in our institute. Forty patients (45%) were women. Mean age at the time of TOF repair was 4.4 years (range, 2 to 27 years), mean follow-up period was 24.3 years (range, 10 to 47 years), and a total of 2,163 patient-years were assessed. Thirty-three patients (37%) had previously undergone palliative procedures before complete repair for TOF. Type of TOF repair was TAP repair in 53 (60%), trans-right ventriculotomy (RV-tomy) in 35 (39%) and trans-right atrial and pulmonary artery repair (RAPA) in 1 (1%) patient. Patient characteristics are summarized in Tables 1,2.
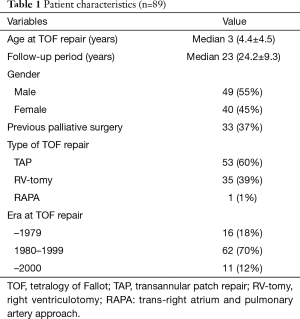
Full table
Full table
Clinical variables
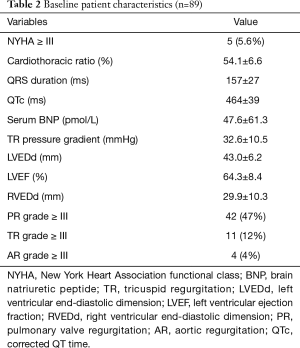
Demographic variables, surgical records, current status and information about arrhythmia were obtained from the hospital records and clinical visits. Detected ventricular tachyarrhythmia [sustained ventricular tachycardia (VT) and ventricular fibrillation (VF)] and SCD were the primary endpoints of this study. If a patient with previous detected ventricular tachyarrhythmia suddenly died, the patient was classified as having SCD. Electrocardiography (ECG) measurement of maximum QRS duration and corrected QT duration was analyzed manually from standard (25 mm/s and 1 mV/cm) 12-lead sinus ECG. Change rate of QRS duration during the study was calculated as the absolute change of the maximum QRS duration divided by the number of observed years. Cardiothoracic ratio from the posteroanterior chest radiograph was used as an estimate of cardiac size. The right ventricular systolic pressure (calculated from the velocity of the tricuspid regurgitation), peak instantaneous systolic pressure gradient across the right ventricular outflow tract (RVOT), end-diastolic dimension of the right and left ventricle (RVEDd and LVEDd), left ventricular ejection fraction and severity of tricuspid, pulmonary and aortic regurgitation were recorded from echocardiogram.
Statistical analysis
Data are expressed as mean ± standard deviation for normally distributed continuous variables or as median for skewed continuous variables, according to the Shapiro-Wilk test. Testing for differences in demographic and clinical data was accomplished using the unpaired Student t-test, Mann-Whitney’s U-test or Kruskal-Wallis test for continuous variables and using Fisher’s exact test for categorical variables, as appropriate. The Kaplan-Meier method was used to analyze long-term survival and freedom from late arrhythmia. Statistical significance was defined as P<0.05. Multiple logistic regression analysis was used to identify risk factors for VT and fibrillation and SCD. Variables with a value of P<0.1 by univariate analysis were entered into a multiple logistic analysis model. Statistical analysis was done using SPSS statistical software (Version 16.0, SPSS, Inc., Chicago, IL, USA).
Results
Survival and late period intervention
The overall survival rate at 20, 30 and 40 years was 100%, 94.6%, 94.6%, respectively. Three patients (3.4%) died in the late period (due to cerebral hemorrhage in 1 patient, due to SCD in 2 patients). Surgical re-intervention was performed in 8 patients (9.0%), including pulmonary valve replacement or RVOT procedure in 4 patients, aortic valve replacement in 2 patients, tricuspid valve surgery in 1 patient, and coronary artery bypass grafting in 1 patient.
Contribution of TAP repair
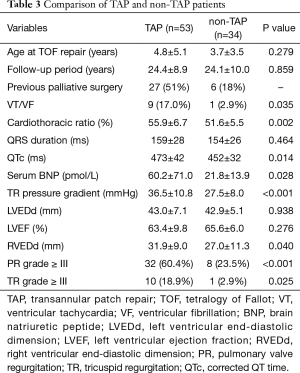
Fifty-three patients had undergone TAP, and the remaining 36 patients had undergone non-TAP repair: RV-tomy repair and RAPA. We compared the TAP patients and non-TAP (RV-tomy and RAPA) patients. There was no difference of age at TOF repair or in follow-up period duration between the two groups. There was no significant difference in long-term survival rate with or without TAP repair according to the log-rank test (P=0.700). In TAP patients, the incidence of VT or VF was higher (17.0% vs. 2.9%; P=0.035) and the cardiothoracic ratio was larger (55.9%±6.7% vs. 51.6%±5.5%; P=0.002) than those of non-TAP patients. According to log-rank test, freedom from VT/VF rate was significantly higher in TAP patients than those in no-TAP patients (P=0.035). On ECG, QRS duration was not different, though QTc duration was longer in TAP patients (473±42 vs. 452±32 ms; P=0.014). Serum brain natriuretic peptide level was higher (60.2±71.0 vs. 21.8±13.9 pmol/L; P=0.028) in TAP patients. On echocardiography, the incidence of pulmonary valve regurgitation (PR) more than grade III and tricuspid regurgitation more than grade III were significantly higher in TAP patients (60.4% vs. 22.9%; P<0.001 and 18.9% vs. 2.9%; P=0.025). Pressure gradient at TR was larger (36.5±10.8 vs. 27.5±8.0 mmHg; P<0.001) and RVEDd was larger (31.9±9.0 vs. 27.0±11.3 mm; P=0.040) in TAP patients. For both TAP and non-TAP patients, left ventricular ejection fraction and LVEDd were not significantly different (Table 3).
Full table
Risk factors for late ventricular tachyarrhythmia
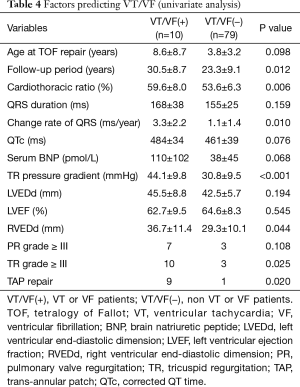
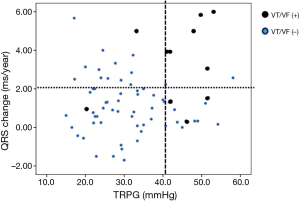
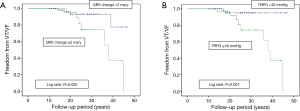
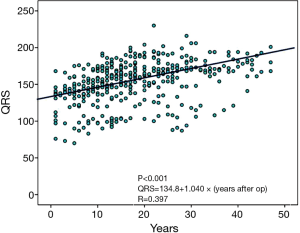
Ten patients experienced sustained VT or VF: 9 (17%) among the TAP patients and 1 (3%) among the non-TAP patients. Six patients required implantation of an ICD. In univariate analysis comparing VT/VF patients and non-VT/VF patients, VT/VF patients had a higher ratio of TAP repair (17% vs. 3%; P=0.020), a longer follow-up period (30.5±8.7 vs. 23.3±9.1 years; P=0.012), a larger cardiothoracic ratio (59.6±8.0% vs. 53.6±6.3%; P=0.006), and a higher change rate of QRS duration (3.3±2.2 vs. 1.1±1.4 ms/year; P=0.010). When echocardiography results of TAP and non-TAP patients were compared, larger RVEDd (36.7±11.4 vs. 29.3±10.1 mm; P=0.044) and a larger pressure gradient at TR (44.1±9.8 vs. 30.8±9.5 mmHg; P<0.001) were seen in VT/VF patients (Table 4). Multivariate analysis showed that change rate of QRS duration (ms/year: P=0.007, HR 2.44 and 95% CI: 1.28–4.65) and pressure gradient at TR (mmHg: P=0.017, HR 1.12 and 95% CI: 1.02–1.22) were recognized as predictive risk factors for late occurring of VT/VF (Table 5). VT/VF was frequently seen in patients combined with both higher TR pressure gradient and higher change rate of QRS duration (Figure 1). Freedom from VT/VF was lower in patients with QRS change rate more than 2 ms/year than those with change rate less than 2 ms/year (P=0.025). In patients with pressure gradient at tricuspid regurgitation more than 40 mmHg, freedom from VT/VF was lower than those with pressure gradient less than 40 mmHg (P=0.007) (Figure 2A,B). There was weak correlation between the change rate of QRS duration and follow-up period after TOF repair [P<0.001; QRS =134.8+1.040× (years after TOF repair); R=0.397] (Figure 3).
Full table

Full table
Discussion
The present study demonstrated that the early change rate of QRS duration and higher pressure gradient at TR, but not TAP repair, could predict the occurrence of late ventricular tachyarrhythmia. The combination of these two factors was strongly related to late ventricular tachyarrhythmia.
Although the long-term outcomes and quality of life of patients who have undergone TOF repair have improved in the recent era, the survival rate is not still the same as for age- and sex-matched controls, mainly because of the risk of ventricular tachyarrhythmias and SCD in the late period (2,8,9). The incidence of SCD was reported to be between 1.5 and 4.5 per 1,000 patient years, SCD tends to occur at more than 4 years after TOF repair. Ventricular ectopic contractions occur in 19% in surgically managed TOF patients and are associated with an increase in the rate of SCD (10). Therefore, identification of patients who are at high-risk patients for life-threatening arrhythmias, and adequate prevention and treatment (including pulmonary valve replacement, electrophysiological ablation and implantable defibrillators) for these patients is crucial.
Various studies have identified risk factors for SCD and ventricular arrhythmias. Having a diagnosis of TOF in itself is a risk factor for SCD, because SCD occurs more frequently in patients with TOF who do not undergo surgical repair (11). Older age at TOF repair is a strong risk factor for SCD and VT (11). Numerous reports had demonstrated that a past history of palliative surgery and TAP repair are risk factors for SCD. A right ventricular incision is also associated with high risk of ventricular arrhythmia and SCD, since the site of surgical scar is believed to provide a substrate for abnormalities of depolarization and repolarization of the myocardium. Some investigators have described a relationship between ventricular tachyarrhythmias and right ventricular dilatation due to chronic volume load by means of PR. Furthermore, several ECG parameters, including QRS duration, change rate of QRS duration, QT/JT duration and dispersion, heart rate variability, and fragmented QRS, have been recognized as risk factors for life threatening ventricular arrhythmias and SCD (1,4,6,7,12,13). Residual cardiac lesions or hemodynamic abnormalities are associated with late SCD, including elevated RV pressure due to RVOT obstruction or pulmonary artery stenosis, right ventricular volume load, right ventricular dysfunction, left ventricular longitudinal dysfunction, and left ventricular diastolic dysfunction (5,14). Despite these studies, no single risk factor has yet been identified to predict VT and SCD in patients who have undergone TOF repair.
Some investigators have concluded that TAP repair is a risk factor for late RV dilatation, ventricular arrhythmias and SCD in patients who have undergone repair of TOF (15,16). TAP repair provides excellent relief of the RVOT obstruction in TOF patients, but it can results in PR. In fact, excess enlargement of the RVOT may cause a significant amount of the PR. However, some investigators demonstrated that TAP repair in itself does not result in a worse late functional outcome than other types of repair in which the RV incision is limited to the ventricle (17). Those investigators argue that both TAP repair and RV-tomy repair are responsible for a similar degree of long-term PR and the same degree of the RV dilatation. In the present study, the incidence of VT and SCD was higher in TAP patients, but TAP repair was not recognized as a predictive factor for VT and SCD within multivariate analysis.
Prolonged QRS duration is closely related to PR and cardiac size, especially in the context of RV dilatation, and it is one of the most important risk factors for VT/VF and SCD in patients who have undergone TOF repair (1,4,7). Prolongation of QRS duration on ECG is frequently recognized after TOF repair, due to surgical injury of the myocardium, right bundle branch block immediately after corrective surgery, and right ventricular dilation caused by volume overload from pulmonary or tricuspid valve regurgitation late after repair (3,18). Therefore, QRS duration is affected by both early and late factors and is probably determined by type of surgical intervention. If complete right bundle branch block (CRBBB) or relatively large RV scarring occurred at the time of TOF repair without significant postoperative PR, the QRS duration would be prolonged, but right ventricular dilatation would not progress. In such cases, life threatening ventricular arrhythmias in the late period is rare. By contrast, if QRS duration was not significantly prolonged with a large amount of PR after TOF repair, late RV dilatation would cause prolongation of QRS duration and probably induce VT/VF. Based on these data, the change rate of QRS duration is believed to be more sensitive and to be a predictive risk factor for VT/VF when compared with the absolute QRS duration. These findings could differ according to the treating center, since the size of RV-tomy and the incidence of CRBBB might differ among institutes and different surgeons. Further, surgical techniques may differ among different hospitals, especially with regard to differences in the range of ventricular incision and muscle resection of the RVOT.
Elevated right ventricular pressure is a cause of fatal ventricular arrhythmias late after TOF repair in some studies. A previous study revealed that RV systolic pressure more than 60 mmHg is a risk factor for ventricular arrhythmias and that residual RV to PA outflow gradient of more than 40 mmHg is a risk factor of SCD (19). However, other investigators suggest that the residual pressure load of RV is not a risk factor for SCD, and some studies have demonstrated that adequate RVOT obstruction might be advantageous against PR (20). It is still controversial whether or not elevated RV pressure is a risk factor for SCD. The TR pressure gradient is thought to be more sensitive for elevated RV systolic pressure than flow velocity at RVOT, since the obstruction might occur at a more distal point than the RVOT (e.g., distal pulmonary artery stenosis), and this variable was also recognized as a predictive risk factor for late VT/VF in patinets with repaired TOF.
Gatzoulis et al. reported that moderate-to-severe pulmonary insufficiency combined with right ventricular outflow obstruction is a risk factor for SCD or life threatening ventricular arrhythmias late after TOF repair (3). In the present study, the combination of rapid change in the QRS duration and higher-pressure gradient at tricuspid regurgitation was strongly related to late arrhythmia and SCD. In other words, the combination of pressure load and volume load of the right ventricle may be used to predict the risk of SCD and VT/VF and may be more powerful for this purpose than the use of either factor alone. Therefore, if adequate surgical or catheter intervention for patients complicated with combination of pressure and volume load for the RV, the risk of late VT/VF could be decreased in patients with repaired TOF.
Magnetic resonance imaging is the best modality to measure right ventricular volume but is expensive and time-consuming (21). Echocardiography and ECG is non-invasive and readily available, but these two parameters (change rate of QRS duration and pressure gradient at tricuspid regurgitation) are quite simply determined and are available from routine examination. Therefore, we conclude that these factors (change rate of QRS duration and pressure gradient at tricuspid regurgitation) are easily assessed and are informative for prediction of late VT/VF in patients who have undergone TOF repair.
This study has several limitations. This study was retrospective, and the surgical technique varied during the study period. Holter ECG was done in only a small portion of the patient cohort, and arrhythmias were diagnosed by standard 12-lead ECG performed at an outpatient clinic. Clinical data was only collected from patients who survived a pre-specified amount of time. Furthermore, this study did not include all patients during the study period, and a relatively large number of patients did not satisfy the follow-up duration criteria needed for inclusion in this study because some patients were followed by other hospitals or they were lost to follow-up. However, we believed that these factors did not significantly affect the results of this study.
Conclusions
The combination of larger change rate of QRS duration and higher-pressure gradient at tricuspid regurgitation is a risk factor for ventricular tachyarrhythmias and SCD late after TOF repair. Adequate surgical or catheter intervention for pressure and volume load in the right ventricle might decrease the incidence of VT/VF and SCD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Niigata University Ethics Committee (No. 2017-0250) approved this retrospective study, and the need for informed consent was waived.
References
- Khairy P, Aboulhosn J, Gurvitz MZ, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation 2010;122:868-75. [Crossref] [PubMed]
- Deanfield JE, McKenna WJ, Presbitero P, et al. Ventricular arrhythmia in unrepaired and repaired tetralogy of Fallot. Relation to age, timing of repair, and haemodynamic status. Br Heart J 1984;52:77-81. [Crossref] [PubMed]
- Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356:975-81. [Crossref] [PubMed]
- Gatzoulis MA, Till JA, Somerville J, et al. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995;92:231-7. [Crossref] [PubMed]
- Diller GP, Kempny A, Liodakis E, et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation 2012;125:2440-6. [Crossref] [PubMed]
- Steeds RP, Oakley D. Predicting late sudden death from ventricular arrhythmia in adults following surgical repair of tetralogy of Fallot. QJM 2004;97:7-13. [Crossref] [PubMed]
- Nakazawa M, Shinohara T, Sasaki A, et al. Arrhythmias late after repair of tetralogy of fallot: a Japanese Multicenter Study. Circ J 2004;68:126-30. [Crossref] [PubMed]
- Koyak Z, Harris L, de Groot JR, et al. Sudden cardiac death in adult congenital heart disease. Circulation 2012;126:1944-54. [Crossref] [PubMed]
- Deanfield JE, Ho SY, Anderson RH, et al. Late sudden death after repair of tetralogy of Fallot: a clinicopathologic study. Circulation 1983;67:626-31. [Crossref] [PubMed]
- Silka MJ, Hardy BG, Menashe VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 1998;32:245-51. [Crossref] [PubMed]
- Sullivan ID, Presbitero P, Gooch VM, et al. Is ventricular arrhythmia in repaired tetralogy of Fallot an effect of operation or a consequence of the course of the disease? A prospective study. Br Heart J 1987;58:40-4. [Crossref] [PubMed]
- Shanmugam N, Yap J, Tan RS, et al. Fragmented QRS complexes predict right ventricular dysfunction and outflow tract aneurysms in patients with repaired tetralogy of Fallot. Int J Cardiol 2013;167:1366-72. [Crossref] [PubMed]
- Park SJ, On YK, Kim JS, et al. Relation of fragmented QRS complex to right ventricular fibrosis detected by late gadolinium enhancement cardiac magnetic resonance in adults with repaired tetralogy of fallot. Am J Cardiol 2012;109:110-5. [Crossref] [PubMed]
- Aboulhosn JA, Lluri G, Gurvitz MZ, et al. Left and right ventricular diastolic function in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Can J Cardiol 2013;29:866-72. [Crossref] [PubMed]
- Kim H, Sung SC, Kim SH, et al. Early and late outcomes of total repair of tetralogy of Fallot: risk factors for late right ventricular dilatation. Interact Cardiovasc Thorac Surg 2013;17:956-62. [Crossref] [PubMed]
- Park CS, Lee JR, Lim HG, et al. The long-term result of total repair for tetralogy of Fallot. Eur J Cardiothorac Surg 2010;38:311-7. [Crossref] [PubMed]
- d'Udekem Y, Ovaert C, Grandjean F, et al. Tetralogy of Fallot: transannular and right ventricular patching equally affect late functional status. Circulation 2000;102:III116-22. [Crossref] [PubMed]
- Abd El Rahman MY, Abdul-Khaliq H, Vogel M, et al. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 2000;84:416-20. [Crossref] [PubMed]
- Garson A Jr, Randall DC, Gillette PC, et al. Prevention of sudden death after repair of tetralogy of Fallot: treatment of ventricular arrhythmias. J Am Coll Cardiol 1985;6:221-7. [Crossref] [PubMed]
- Yoo BW, Kim JO, Kim YJ, et al. Impact of pressure load caused by right ventricular outflow tract obstruction on right ventricular volume overload in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg 2012;143:1299-304. [Crossref] [PubMed]
- Knauth AL, Gauvreau K, Powell AJ, et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart 2008;94:211-6. [Crossref] [PubMed]


