Pulmonary metastasectomy in sarcoma—experiences with laser-assisted resection
Introduction
Pulmonary metastasectomy is associated with a survival benefit in well selected patients in different tumor types (1-3). Suitable candidates are selected based on clinical observations such as the disease-free interval (DFI), number of metastases and tumor growth rate. Pulmonary metastasectomy can be carried out using different surgical techniques including staplers, cautery devices, Ligasure/Ultracision-System as well as Nd:YAG-lasers (4). The postulated advantage of laser-assisted surgery (LAS) is the possibility to resect more metastases due to minimal loss of healthy lung tissue with similar recurrence rates. Consequently, recent studies have been showing the complete resection of a significantly higher number of metastases compared with conventional resection while achieving similar long-term survival rates (5-7).
Pulmonary metastasectomy in sarcoma patients is still controversial, however, there are retrospective data suggesting a survival benefit in certain patients (8-11). Due to the high recurrence rates in sarcoma, often multiple operations are required, creating the need for sparing surgical techniques. In this study we investigate possible advantages of LAS using a 1,320 nm Nd:YAG-laser specifically in pulmonary metastasized sarcoma patients.
Methods
Data were extracted from a prospectively maintained institutional database. A total of 83 patients who underwent pulmonary metastasectomy at our clinic during the years 2005 to 2016 were identified. LAS was carried out using a 1,320 nm diode-pumped Nd:YAG-laser (Limax® 120, Gebrüder Martin GmbH & Co. KG, Tuttlingen, Germany). Inclusion criteria were pulmonary metastasized bone- or soft-tissue sarcoma, no sign of local recurrences or extrathoracic metastases at the time of surgery, adequate cardiopulmonary reserve to undergo major thoracic surgery and intended R0-resection. Seven patients in whom the first pulmonary metastasectomy was not performed at our institution were excluded from further analysis. The study was approved by our local ethics committee and registered in the German Registry for Clinical Trials (DRKS-ID: DRKS00010272). Informed consent was waived due to the retrospective nature of the study.
Data were recorded in a database designed in Microsoft Office Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 7.01 (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis. If not stated differently, continuous data were reported as median with interquartile range (IQR). Categorical and count data were presented as frequencies and percentages. Data sets were tested for normality using the D’Agostino-Pearson omnibus normality test. In normally distributed data-sets Student’s t-test, in non-normally distributed data-sets Mann-Whitney-test was performed. Categorical variables were tested for dependency using Fisher’s exact test and the odds-ratio (OR) and the associated confidence intervals were calculated.
For evaluation of survival data the Kaplan-Maier estimator was employed and the log-rank test was used for comparison of survival curves. Results were considered statistically significant if the P value was less than 0.05. A P value between 0.05–0.1 was considered a trend. Overall survival (OS) was defined as the time interval from surgery to death by any cause. Disease-free survival (DFS) was defined as the time interval from surgery to tumor recurrence or death by any cause. The DFI was defined as the time from resection of the primary to first surgery on pulmonary metastases. Long and short DFI was defined by using the median as a cut-off point.
Results
Clinical and surgical data
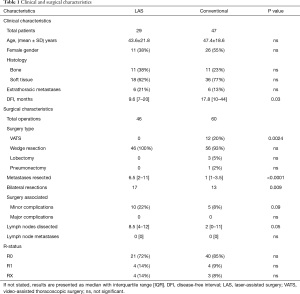
A total of 106 operations were performed in 76 patients. LAS was performed in 46 and conventional resection in 60 cases. The mean age at the time of surgery was 43.6±21.8 in the LAS group and 47.4±18.6 in the conventional group and there were 11 (38%) and 26 (55%) female patients in the respective cohorts.
In LAS surgical approach was exclusively sublobar wedge resection via thoracotomy. In the control group 3 lobectomies and 1 pneumonectomy were carried out for resection of the metastases and in 12 patients minimally-invasive thoracoscopic surgery was performed.
Significantly more patients were treated bilaterally with LAS (OR =3.7, P=0.009). Also, there was a trend towards more minor surgery associated complications, including pneumonia (n=3), empyema (n=1), persistent air leakage (n=4) requiring re-insertion of a chest tube in one case and of re-operation in two cases and hemothorax, also requiring re-operation in two cases (OR =3.1, P=0.09). Mean hospital stay was comparable at 9 (IQR, 7–9) days in the LAS and 7.7 (IQR, 5.5–9.8) days in the conventional group. No major complications occurred and there were no surgical mortalities in either cohort. Repeated surgery was performed in either combined or isolated pulmonary relapse in the LAS group in 7 cases and in the conventional group in 12 cases.
Significantly more metastases were resected in the LAS group compared to the conventional group [6.5 (IQR, 2.0–11.0) vs. 1.0 (IQR, 1.0–3.5); P<0.0001]. Lymph node sampling was carried out in 50 (66%) patients and in 5 (7%) lymph node metastases were present. In patients treated by LAS the lymph node dissection rate was higher (OR =2.8, P=0.08) and more lymph nodes were dissected [8.5 (IQR, 4.0–12.0) vs. 2.0 (IQR, 0–11.0), P=0.05]. Positive resection margins (R1) could be found in 4 (9%) cases in the LAS and in 4 (7%) cases in the conventionally resected cohort. Extrathoracic metastases were present in 6 (21%) and 6 (13%) patients in the respective groups. Clinical and surgical data are summarized in Table 1.
Full table
Survival analysis
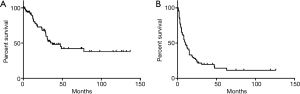
The median OS was 37 months and DFS 10 months respectively (Figure 1). Median survival in the LAS group was 77.6 months and 29.0 months in the conventional group [hazard ratio (HR) =0.74, P=0.4269] (Figure 2). The 2- and 5-year survival rates were 71% and 63% as well as 53% and 36% in the respective cohorts. There were 64% tumor recurrences in the LAS group and 58% in the conventional group (OR =1.3, P=0.6). DFS was also comparable in the respective groups (HR =1.6, P=0.15).
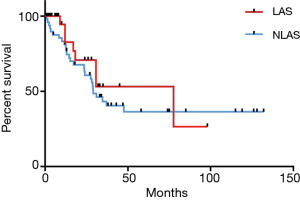
In patients in whom 5 or more metastases were removed median survival was significantly worse than in patients with fewer metastases (23.7 vs. 77.6 months, HR =3.2, P=0.0035). When comparing patients with 5 or more metastases in the subgroup of LAS no statistically significant survival difference can be seen (17 vs. 30 months, HR =1.6, P=0.33). Furthermore, patients in whom pulmonary relapse was treated by repeated pulmonary metastasectomy compared to those who were treated by chemo- and/or radiotherapy showed longer OS (HR =0.3, P=0.01) (Figure 3).
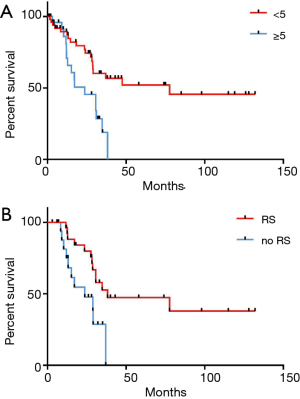
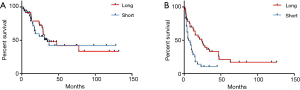
DFI was significantly shorter in the LAS group (9.6 vs. 17.8 months, P=0.03). Comparison of OS in patients with a shorter and longer time period between resection of the primary tumor and the first pulmonary metastasectomy did not show significant differences (P=0.77). However, the DFI after resection of the primary was a prognosticator for shorter DFS (HR =2.7, P=0.001) (Figure 4). Interestingly, age was neither a predictor for OS nor DFS. Furthermore, neither presence of lymph node metastasis nor resection status were prognosticators for OS or DFS.
Discussion
The majority of metastasized sarcoma patients develop lung metastasis and in about 60% of cases metastases are confined to the lung. If respective surgical and oncologic conditions apply, pulmonary metastasectomy has become standard of care and multiple studies have shown survival benefit in patients who underwent resection of metastases (1-3,12,13). Despite advances in systemic and non-surgical local ablative treatments, tumor relapse remains common in metastasized sarcoma and often multiple operations are required. Notably, in case of recurrence and given resectability, performing repeated resections has been associated with a prolonged survival (10,11). Accordingly, in our study cohort patients in whom tumor relapse was treated by repeated resections showed longer OS.
To enable the treating oncologic surgeon to carry out multiple pulmonary metastasectomies several requirements have to be met: complete resection of the metastases, preservation of the lung tissue and as little damage to the adjacent lung tissue as possible, including deformation which could lead to occlusion of the bronchi and consecutive atelectasis.
Recent series have suggested LAS with 1,320 nm Nd:YAG-lasers to be parenchyma-sparing, improve complete resection rates and thus provide beneficial results with longer survival rates especially in the case of resection of multiple pulmonary metastases (7,14). LAS provides a high precision tool for resection, causing only minimal damage and deformation of the surrounding lung tissue (15). In a recent study by Welter and colleagues which focused on different growth patterns of resected metastases depending on the histologic subtype, histologic analysis also showed that laser resection was carried out with the closest resection margins, however, not resulting in higher recurrence rates (16). Furthermore, laser resection is thought to provide better sealing of the lung tissue and coagulation of the intrapulmonary vessels resulting in a shorter air leakage time and hospital stay (17). Given these characteristics LAS provides ideal features for the requirements in pulmonary metastasized sarcoma.
Nevertheless there are some drawbacks regarding the use of LAS for pulmonary metastasectomy as there are less costly and easier techniques for resection. For example precision cautery is a simple and inexpensive technique that allows the surgeon to spare the parenchyma. Consequently there is more evidence needed to justify the use of LAS and provide possible explanations for better results.
In this study we aimed to specifically investigate laser-assisted pulmonary metastasectomy in sarcoma patients. To our knowledge there has only been one small series on ten patients which studied LAS in pulmonary metastasized sarcoma, showing a survival benefit in patients which were treated using LAS (18). Going in line with these findings we show similar OS rates in both groups despite a significantly higher number of metastases resected using LAS. The number of metastases is one of the main factors predicting survival in surgery for pulmonary metastasis in general and sarcoma patients in particular. Depending on the study more than one, two or more than four metastases have been associated with significantly worse survival (10,11). Further common prognosticators after surgical therapy of pulmonary metastasis include the time from resection of the primary tumor to detection of the metastasis, complete resection and presence of extrathoracic metastases (8,9,19). Also, in some studies if three or fewer metastases were present female sex has been associated with a favorable prognosis (20). All these characteristics are comparable in the respective groups or in favor of the non-LAS group and thus should not influence the observed results. At this point one can only speculate why patients would benefit from LAS. Recurrence rates remain high and there is a tendency to a shorter DFS in the LAS-Group, which however does not result in a worse OS. Accordingly, in the whole cohort shorter DFI results in a worse DFS but not OS. Aggressive local treatment in pulmonary metastasized sarcoma seems to result in a longer OS whilst not influencing tumor biology. LAS constitutes a tool which enables the treating oncologic surgeon to perform the often required repeated resections of multiple metastases and could thus be beneficial.
In the current guidelines by the European Society for Medical Oncology (ESMO) pulmonary metastasectomy in soft tissue sarcoma is recommended only in patients with a DFI >1 year without signs of extrathoracic metastases in whom an R0-resection can be achieved (21). In the LAS-group there were six patients who presented with extrathoracic metastases during the course of disease. However, at the time of resection of the pulmonary metastases these were controlled by local measures, thus confining the disease to the lungs. In general, treatment modalities should always be discussed in a multidisciplinary setting, as clinical courses in sarcoma patients are highly variable.
Regarding the surgical technique thoracoscopic approaches have been shown to provide similar results if two or fewer metastases are present (22). A possible advantage is thought to be the preservation of the possibility of repeated resections. In our institution, video-assisted thoracoscopic surgery (VATS) is performed if there are up to two peripherally located solitary metastases, in all other cases open resection is carried out. In the study at hand in 12 cases in the non-LAS group, the thoracoscopic approach was performed. LAS was always carried out via thoracotomy and metastases were resected via wedge resection or vaporization. Although minimally-invasive techniques for LAS have also been described the standard approach remains open surgery (23). Notably, we found slightly more minor surgical complications which occurred in the LAS-group, which, however, had no influence on the length of the hospital stay and surgical outcome. Possibly the much higher numbers of metastases which were resected via LAS are responsible for this finding. However, minimally invasive procedures are associated with fewer complications and were exclusively carried out in the conventional group, hence providing an alternative explanation for this observation.
Limitations
Limitations of this study include the retrospective nature and mixing the entities of soft-tissue and osteosarcoma. Also, the decision on whether to perform LAS or conventional resection was not standardized and was based on surgeon’s preference, localization, number of metastases and logistic reasons. Moreover, not least based on the analysis of our own data, standard approach at our institution for resection of multiple metastases has been LAS in the recent years. The thoracoscopic approach is limited to cases with one or two unilateral metastases. Nevertheless, as they represent mere observations presented data should be interpreted cautiously and no definitive conclusions can be drawn.
Conclusions
In this single-institution series we present first data on LAS in pulmonary metastasized sarcoma. Our main finding, that LAS provides similar overall survival rates with resection of significantly more metastases, goes in line with other recent series. Expectably, recurrence rates in metastasized sarcoma patients remain high with either surgical method. Future studies will have to evaluate possible advantages provided by LAS in a prospective manner.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our local ethics committee and registered in the German Registry for Clinical Trials (DRKS-ID: DRKS00010272). Informed consent was waived due to the retrospective nature of the study.
References
- Kaifi JT, Gusani NJ, Deshaies I, et al. Indications and approach to surgical resection of lung metastases. J Surg Oncol 2010;102:187-95. [Crossref] [PubMed]
- Younes RN, Fares AL, Gross JL. Pulmonary metastasectomy: a multivariate analysis of 440 patients undergoing complete resection. Interact Cardiovasc Thorac Surg 2012;14:156-61. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Venuta F, Rolle A, Anile M, et al. Techniques Used in Lung Metastasectomy. J Thorac Oncol 2010;5:S145-50. [Crossref] [PubMed]
- Baier B, Kern A, Kaderali L, et al. Retrospective survival analysis of 237 consecutive patients with multiple pulmonary metastases from advanced renal cell carcinoma exclusively resected by a 1318-nm laser. Interact Cardiovasc Thorac Surg 2015;21:211-7. [Crossref] [PubMed]
- Osei-Agyemang T, Palade E, Haderthauer J, et al. Pulmonary metastasectomy: an analysis of technical and oncological outcomes in 301 patients with a focus on laser resection. Zentralbl Chir 2013;138 Suppl 1:S45-51. [PubMed]
- Franzke K, Natanov R, Zinne N, et al. Pulmonary metastasectomy - A retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur J Surg Oncol 2017;43:1357-64. [Crossref] [PubMed]
- García Franco CE, Algarra SM, Ezcurra AT, et al. Long-term results after resection for soft tissue sarcoma pulmonary metastases. Interact Cardiovasc Thorac Surg 2009;9:223-6. [Crossref] [PubMed]
- García Franco CE, Torre W, Tamura A, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg 2010;37:1205-8. [Crossref] [PubMed]
- Mizuno T, Taniguchi T, Ishikawa Y, et al. Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment? Eur J Cardiothorac Surg 2013;43:795-9. [Crossref] [PubMed]
- Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011;92:1780-6; discussion 1786-7.
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [Crossref] [PubMed]
- Pfannschmidt J, Egerer G, Bischof M, et al. Surgical intervention for pulmonary metastases. Dtsch Arztebl Int 2012;109:645-51. [PubMed]
- Rolle A, Koch R, Alpard SK, et al. Lobe-sparing resection of multiple pulmonary metastases with a new 1318-nm Nd:YAG laser--first 100 patients. Ann Thorac Surg 2002;74:865-9. [Crossref] [PubMed]
- Kirschbaum A, Braun S, Rexin P, et al. Comparison of local tissue damage: monopolar cutter versus Nd:YAG laser for lung parenchyma resection. An experimental study. Interact Cardiovasc Thorac Surg 2014;18:1-6. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tonini G, et al. Pulmonary metastasectomy: might the type of resection affect survival? J Surg Oncol 2001;76:47-52. [Crossref] [PubMed]
- Ueda T, Uchida A, Kodama K, et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer 1993;72:1919-25. [Crossref] [PubMed]
- Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg 2009;88:877-84; discussion 884-5. [Crossref] [PubMed]
- Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009;35:356-61. [Crossref] [PubMed]
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii102-12. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- 23 Meyer C, Bartsch D, Mirow N, et al. Video-Assisted Laser Resection of Lung Metastases-Feasibility of a New Surgical Technique. Thorac Cardiovasc Surg 2017;65:382-6.