Biomarkers in the prevention and follow-up of workers exposed to asbestos
Introduction
Asbestos is a natural fibrosus mineral with many useful properties from the industrial point of view. It is extremely durable, resistant to most chemical reactions as well as fire and hot temperatures. Consequently, asbestos, mined for centuries, has been increasingly used for a variety of applications around the world, especially heavy industry (steelmaking, siderurgic and metallurgic implants, chemical, electrical, automotive industry, etc.) and construction activities. Worldwide commercial production of asbestos reached a peak of more than 5 million tons in the 1970s and WHO estimates that 125 million workers are exposed to asbestos (1). Many domestic tools or products are responsible for a non-occupational exposure to asbestos, too. Asbestos is nowadays banned in most developed countries, but there is a consistent part of the world where mine extraction, trade and use in manufacturing is still allowed, such as Canada, Russia, China, Brazil. Due to its long bio-persistence, it continues to be responsible both for a number of benign and malignant diseases. Asbestosis, pleural plaques, diffuse pleural thickening, round atelectasis and bronchiectasis are the common benign asbestos-related lung diseases. Inhaled asbestos fibers cause a number of malignancies such as mesothelioma (pleural, pericardial, peritoneal), lung cancer, larynx cancer, ovarian cancer, and although with less extent of evidence, it has been associated to some gastro-intestinal cancers, too. However, among these cancers only malignant pleural mesothelioma (MPM) is demonstrably caused by asbestos at as high as more than 80% cases, after a latency period of decades. This has oriented the scientific investigation toward the research on biomarkers potentially useful for preventive or clinical purposes, mainly targeting MPM.
Biomarkers classification
According to the National Cancer Institute Dictionary of Cancer Terms, biomarkers are biological molecules found in blood, other body fluids, or tissues that are a sign of a normal or abnormal process, or of a condition or disease. Another highly referenced definition was expressed by Hulka and colleagues in the nineties (2): “cellular, biochemical or molecular alterations that are measurable in biological media such as human tissues, cells, or fluids.” Biological markers (biomarkers) lend themselves to a wide range of applications. They are currently used in both fields of prevention (biomarkers of exposure, risk prediction, etc.) and clinical investigation (biomarkers of early diagnosis, prognosis, treatment response, etc.) and potentially track all the steps from the exposure to an etiological factor to the complete evolution of a disease. The classification in “biomarker of exposure”, “effect” and “susceptibility” is well established. Under the category of “biomarkers of exposure” fall all those molecules, which provide information regarding the dose of the exposure, which in turn is usually related to the potential for adverse effects on health.
“Biomarkers of effect” are biological indicators of the body’s response to exposure. They usually represent from early to late sub-clinical effects, as expression of the involvement in some pathogenic pathways. Most biomarkers studied to date belong to this category. They often result from the attempt to dose in blood those molecules responsible for the specific cancer histotype, sometimes even with no clear evidence of any pathogenic specific role. The majority of markers of diagnosis, early diagnosis, prognosis and response to treatment fall under this latter category. “Biomarkers of susceptibility” indicate subjects with increased tendency to get a health damage after having exposed to specific aetiological factors. They may be specific genetic fingerprints (i.e., defective glutathione S-transferase haplotype haplotype) as well as specific protein patterns often unveiling some individual metabolic deficiency. Of course, classification into these three categories serves much more for didactic than practical purposes, and some molecule’s roles may overlap.
Preventive programs on asbestos exposed population
From a preventive point of view people at high risk for cancer can be targeted through two different approaches: screening programs and health medical surveillance.
In general, screening aims to diagnose cancer or its precursor lesions in apparently healthy, asymptomatic people by means of tests (e.g., fecal occult blood test for colon-rectum cancer), examinations (e.g., Pap test for cervical cancer), imaging (e.g., mammography for breast cancer) or other procedures that can be applied rapidly, and widely accessed by the target population. The diagnosis of asymptomatic early staging cancer is usually the unique objective of the entire program. On the contrary, with “health medical surveillance”, we refer to a system of ongoing different health checks with a wider range of scopes. In the case of asbestos workers objectives of follow-up programs should be not only the early diagnosis of lung malignancies, but also the diagnosis of benign asbestos-induced lung diseases as well as any other clinical (e.g., prompting smoking cessation, influenza and pneumococcus vaccination, etc.), medico-legal (e.g., compensation) or epidemiological purposes. According to the ATS guidelines, to identify the onset of benign thoracic asbestos-related diseases, people with a minimal 10-year long history of asbestos exposure even with no apparent disease, should undergo a preventive follow-up with chest films and pulmonary function respiratory tests every 3 to 5 years (3). Although many asbestos-exposed workers around the world have been periodically in the past and are currently followed-up by chest X-ray and computed tomography (CT) examinations, there still is inadequate evidence of any effectiveness in thoracic cancer diagnosis. So far, screening for thoracic malignancies using periodic chest X-ray or CT in exposed people was not recommended (3,4). High resolution computed or low-dose tomography has been proposed only as second line diagnostic procedure whenever the chest film was altered. However, recent demonstration by the National Lung Screening Trial (NLST) (5) of a reduction in the mortality rate for lung cancer in high risk former or current smokers followed up with annual low-dose CT (LDCT), suggests that individuals who are at or above the risk threshold set for participation in the NLST, regardless the nature of the risk (either determined exclusively by asbestos or by the more than additional effect of combined exposure to asbestos and smoking), might benefit of being enrolled in dedicated follow-up programs (Helsinki criteria 2014) (6). Periodic protocol of radiologic exams should be set on a risk/benefit balance, considering both intrinsic risk for health caused by imaging and the underlying cancer risk due to asbestos exposure and possibly other factors (i.e., smoking habits).
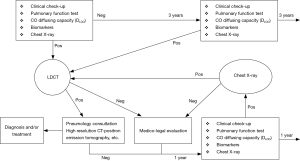
An explicative example of health surveillance applied to a high risk for asbestos exposure population was reported by Guglielmi et al. in 2012 (7), shown in Figure 1. This health surveillance protocol is based on a detailed asbestos-related work history data collection, aimed at a risk stratification, respiratory function tests, thoracic X-ray and/or LDCT. Along with these exams a research program for evaluating the role of some serum biomarkers is performed. The frequency of radiological exams and marker dosage may change according to the trend (positive or negative) of the previous clinical test.
Updated research on biomarkers for MPM
Mesothelin, one of the most investigated markers in the MPM, is a 40-kDa protein expressed at a low level by pleural, peritoneal, pericardial normal mesothelial cells. The term “soluble mesothelin-related peptides” (SMRP) refers to a number of similar proteins deriving from subsequent cleavages of cell-surface precursor. SMRP has been found to be able to discriminate between epithelioid MPM histotype (8), and controls with a high specificity (80–85%), though with less consistent sensitivity (60–90%) (9-11). Diagnostic performance of SMRP may be influenced by the prevalence of specific genetic polymorphisms within the MSLN gene. In fact, some variants both in the promoter (12) and in the 3' untranslated region (13) of the MSLN gene were found to be associated with high levels of SMRP in healthy subjects.
Careful interpretation, before the attribution of a diagnostic valence of SMRP values, is also suggested by the evidence that some clinical conditions such as renal failure or hypertension (14), body mass index (15) may significantly affect diagnostic ability to differentiate MPM from healthy people.
Testing 219 serum samples from 56 MPM patients, collected during their clinical follow-up, we demonstrated (16) that SMRP serum levels parallel (as well as vimentin) the disease status according to response therapy or disease progression (17). Nevertheless, the role of mesothelin in cancer as well as normal cells is not yet clear, even though some evidence was reported that it might promote tumor invasion (18). Recent studies highlight three mechanisms by which mesothelin might play a role in cancer progression. First, mesothelin may aid in the peritoneal implantation and metastasis of tumors through its interaction with mucin MUC16 (also known as CA125) (19,20). Second, mesothelin may promote cancer cell survival and proliferation via the NF-κB signaling pathway (21). Finally, mesothelin expression promotes resistance to certain chemotherapy drugs such as TNF-α, paclitaxel, and a combination of platinum and cyclophosphamide (22). It is expressed mainly by MPM but also by pancreatic cancer, ovarian cancer or some breast and lung cancers (23). However, its cancer-specific expression makes mesothelin a potential target for monoclonal antibody therapy.
Osteopontin (OPN) is a glycoprotein over-expressed in several human neoplasms such as lung, breast and colon cancer (24). OPN modulates cell-matrix interactions; high levels correlate with tumor invasion, progression and metastasis. Mean plasma OPN values were significantly higher in MPM patients than in controls and patients suffering with benign respiratory disease (BRD), and no statistically significant difference was found comparing the mean value of controls and BRD groups. Moreover, neither clinical status nor smoking habit could affect the result of the OPN measurement (25). Unfortunately, use of OPN for diagnostic purpose in MPM is still controversial, since it has been validated in some studies (25,26) but not in others (27). A recent review on the diagnostic accuracy of serum OPN in discriminating MPM from controls showed that the pooled sensitivity and specificity were 57% and 81%, respectively overall serum OPN (28) .
The megakaryocyte potentiating factor (MPF) is a 31-kDa cytokine sharing with mesothelin the same coding gene (mesothelin gene MSLN). This gene codes for a 71-kDa precursor protein, releasing, after a furin cleavage, both 40-kDa C-terminal mature mesothelin and the 31-kDa N-terminal mature MPF. MPF has been measured in blood samples with the aim to test its ability to diagnose MPM by different research groups (14,29-33), using different ELISA assays based either on commercial or home-produced pairs of monoclonal anti-MPF antibodies. Regardless differences in methods all the studies showed that MPF diagnostic performance is similar to SMRP, while the combination of the two biomarkers has given inconsistent results (14,33,34).
Recently a glycoprotein encoded by the EFEMP-1 gene, named Fibulin-3 was suggested as a promising diagnostic marker for MPM (35). Since this first publication, other research groups confirmed the ability of the molecule to discriminate MPM from controls, though the test performance was not reproduced as high as well. A recent meta-analysis conducted by Pei et al. (36), including six studies, 468 MPM patients and 664 controls, reported a pooled sensitivity of 62% at a specificity of 82% after the outlier-adjustment.
High mobility group box 1 (HMGB1) is a very interesting molecule with great potential for an application as a biomarker for diagnostic, prognostic purposes and as exposure biomarker as well. Yang et al. (37) showed that asbestos fibers induce HMBG1 translocation from the nucleus to the cytoplasm and into the extracellular milieu, which in turn is induces macrophages to secrete TNF-α, contributing to the inflammatory response involved into the asbestos carcinogenesis. Other studies provided further mechanisms of inflammatory response via Nalp3 inflammasome activation and IL-1b secretion (38-40). The observation that the inhibition of HMGB1, either by monoclonal antibodies (41) or by ethyl pyruvate is able to suppress the malignant phenotype of cultured mesothelioma cells (42), supports a strategic role of this molecule in the tumor progression. Serum total HMBG1 (i.e., the sum of both acetylated and non-acetylated HMBG1) levels in MPM patients were found significantly higher than in asbestos either exposed or non-exposed individuals (37,43), supporting a role a diagnostic biomarker. Interestingly, total HMBG1 has also been suggested as a biomarker of exposure, since serum mean levels were significantly higher in asbestos exposed than non-exposed subjects with a high-performance accuracy [area under the curve (AUC): 95% CI] (43). Particularly high was the diagnostic accuracy showed by Napolitano et al. of the hyperacetylated HMBG1, which reached a 100% sensitivity, to make a mesothelioma diagnosis, among 22 cases and 20 healthy volunteers. These results are very promising though need confirmation with larger population.
Most cases of MPM (up to 80–90%) are demonstrably associated with either occupational or environmental exposure to asbestos fibres or other elongated mineral particles, such as asbestiform erionite (44). Notwithstanding, multiple cases of MPM in the same family as well as sporadic MPM can occur in the absence of a previous exposure to carcinogenic mineral fibres. Testa et al. (45) discovered that germline mutations in BRCA1-associated protein 1 (BAP1), inherited in an autosomal dominant manner, predispose to MPM. In experimental animals, BAP1 mutations increase the incidence of mesothelioma after exposure to asbestos at very low doses (46). BAP1 might drive to cancer development because of its role in cell-cycle progression and tumour suppressing activity (47,48). The MPM prevalence in experimental model raised up to >70% in BAP1-mutant animals after asbestos exposure, in the face of a spontaneous rate of 2.2% in germinal mutant non-exposed (49). These data suggest that germline BAP1 mutations make individuals more susceptible to develop sporadic MPM and highly increase the asbestos-induced risk of pleural malignancy. In addition, germline BAP1 mutations may be responsible for the almost anecdotic reports of MPM family clustering, as demonstrated in two high-risk mesothelioma families investigated in US (45). A study published in 2013 showed a prevalence of somatic BAP1 mutations in 20% of the analysed MPM (50). These results were replicated 2 years later in 23% of MPM (51). Germline BAP1 mutation can be considered as “marker of susceptibility”, not only for MPM but also for the development of a spectrum of tumour types, including some skin tumours (atypical benign melanocytic lesions, cutaneous melanoma, basal cell carcinoma), uveal melanoma, and some other cancers of the central nervous system, kidney, lung and breast (49).
Micro-RNAs (miRNAs) are, 20–25 nucleotides long, non-coding RNA molecules, which regulate gene expression pairing with complementary sites of the correspondent mRNAs (52). Each mRNA may target large number of genes, and they are involved in many different either physiologic (cell proliferation, differentiation, metabolism, senescence) or pathologic (cancer transformation, invasion, etc.) (53,54). So far, several miRNAs have been suggested as biomarkers for the diagnosis of different type of cancer, including MPM. Although, different authors demonstrated that dysregulated miRNA (both up- and downregulated), detected in both tissues and blood samples, discriminate MPM patients from health controls with significant accuracy. Busacca et al. (55) found miRNA-17-5p, miRNA-21, miRNA-29a, miRNA-30c, miRNA-30e-5p, miRNA-106, and miRNA-143 were differentially expressed in mesothelioma and mesothelial cultured cells. Unfortunately, following research did not reproduce a consistent miRNA signature neither in tissue nor in blood samples. After Santarelli et al. suggested miRNA126 as a blood diagnostic marker for MPM, the same research group confirmed this result (56) also in combination with other biomarkers (57). Bononi et al. in 2016 proposed three circulating up-regulated microRNAs, i.e., miR-197-3p, miR-1281 and miR-32-3p as potential new MPM biomarkers (58). Weber et al. (59) found that the combination of circulating miR-132-3p with miR-126 improved the diagnostic performance of MPM, while miR-20b, miR-28-3p, and miR-146b-5p showed no statistically significant differences between asbestos-exposed controls and mesothelioma patients. De Santi et al. confirmed, after a strong validation test, a significant differentially expression of miR-185, miR-197 and miR-299 in MPM cases and identified a two-miRNA prognostic signature, composed by Let-7c-5p and miR-151a-5p (60).
Although results of studies based on the “miRNomic” approach are very intriguing they lack of sufficient overall consistency. In fact, probably because different microarray panels were tested, different miRNAs from time to time have been proposed. In addition, whenever the same miRNAs have been investigated, differences in methodologies, normalization process, biologic matrix for extraction (serum or plasma), have determined some limitations in their cross-comparison (56). Nonetheless, the available data indicate the probable future utility of miRNAs as biomarkers of prediction, diagnosis, prognosis and, based on in vitro experiment of oncogenic miRNA inhibition and tumor suppressive tumor miRNA substitution, of biomarker of treatment, too.
Multiple biomarkers panels
The need for a reliable biomarker of early diagnosis of MPM has prompted the research around the world since in 2003 when mesothelin was suggested as the first diagnostic biomarker by Robinson et al. in 2003 (61). From that time on several diagnostic biomarkers have been proposed. However, none of them is characterized by the necessary combination of sensitivity and specificity to legitimate a clinical and particularly effective preventive and translation. For this reason, some studies started to investigate the usefulness of combining different biomarkers chosen among most promising ones. One of the first evidence of the goodness of this approach was provided by a study we published in 2011 demonstrating a significant improvement of diagnostic performance by combining serum mesothelin and plasma OPN dosage (62). In fact, the AUC value deriving from the combination of the two markers (0.873) was higher than the one deriving from each of them (0.795 for OPN and 0.62 for SMPR).
Very recently, we carried out a study, which through a preliminary paired genomic and proteomic approach (63,64) allowed us to test in the serum by the use of specific ELISA, a set of putative blood markers for the diagnosis of MPM. Each of these markers were singularly tested for their diagnostic performance as well as in combination with one or more of the other, using a logistic regression method (65). The population enrolled into this study was composed by 43 subjects previously exposed to asbestos and 27 epithelioid MPMs. The best result was given by the combination of six biomarkers: SMRP, plasma OPN, interluekin-6 (IL6), vimentin, desmin, hepatocyte growth factor (HGF). This panel reached at a best cut-off of 0.13 a specificity and a sensitivity of 85.7% and 100%, respectively. So, we concluded that the combination of multiple markers could be very useful rather than the use of single markers in the diagnosis of MPM.
Discussion
The extraordinary long latency along with the possibility of identifying very well-defined populations at high risk for cancer development, as asbestos is almost its unique aetiological factor, make MPM a paradigmatic tumor to test diagnostic, prognostic biomarkers. In addition, sensitive and specific biomarkers for MPM are urgently needed for screening of asbestos-exposed subjects, since early diagnosed patients may benefit a better survival through a more effective multimodality therapy (surgery combined with radiotherapy and chemotherapy) (66,67). Nevertheless, biomarkers for routinely early diagnosis and screening are not available yet. In fact, the SMRP, which is the most widely evaluated serum marker for early diagnosis of malignant mesothelioma, although quite specific, has showed a relatively poor sensitivity. As well, other biomarkers (e.g., OPN, MPF, Hyaluronic acid, etc.) on which attention was driven by most researchers lack adequate either specificity or sensitivity. Some very recently discovered putative mesothelioma biomarkers are very promising (e.g., Fibulin-3, HMGB, miRNAs), displaying a better diagnostic performance in comparison with more “traditional”, but need further confirmation. Notably, HMGB, if confirmed in larger population and other independent research groups, might be very useful also as marker of asbestos exposure. According to the 2014 Helsinki Criteria for Diagnosis and Attribution, some of the studied biomarkers are promising but still need further confirmation through experimentation in larger population and/or in longitudinal screening of asbestos-exposed individuals rather than a single baseline assessment. Consistently with this point of view, the three Italian National Consensus on Mesothelioma, held in Bari in June 2015, stated that though at present the determination of circulating mesothelin is not recommended as a routinely screening tool, it should be encouraged within a research setting. Despite these statements some authors suggest that, especially those makers with particular high specificity, either when are unexpectedly increased or show an increasing trend within serial essays, may aid MPM driving to second level imaging procedures (68). Notwithstanding, it is important to underline the very promising results obtained from the combination of multiple markers for the ability to increase overall performance. However, there are some issues related both to studies design and to the interpretation of biomarkers values in cohorts of asbestos-exposed workers, that worth to be addressed. It is a matter of fact that an early diagnosis test should be characterized by the highest sensitivity and specificity rate as possible. However, it is extremely rare that a single biomarker can accomplish both these requirements, and sensitivity and specificity relate to the test accuracy, but of course, not the probability of the disease. As a general rule of epidemiology, the positive predictive value (PPV), that is the percentage of people positive to the test actually having the disease, will be inevitably low, in the case of rare target disease, even with highly accurate and precise tests. MPM is rare form of cancer in the general population, so it is no reasonable to perform generalized screening campaign for MPM diagnosis. The higher risk of MPM in individuals enrolled for screening, the better will be the PPV. Therefore, a correct classification of asbestos exposure is clearly a crucial point especially in auspicial prospective studied to be performed in order to verify the value of serial biomarkers measurement. Secondly, when tested in at high risk individuals, it is preferable to set up a cut-off point of putative early diagnosis biomarkers, favoring the specificity at the expense of sensitivity, in order to minimize the false positive rate and the consequent psychological distress as well as the increase of unnecessary diagnostic examinations. Finally interpreting biomarkers values we should always take into account all the possible confounding factors (e.g., glomerular filtration, body mass index, specific genetic haplotype, etc.) and most credit has to be given to an increasing trend than to a single occasional increased value. Since MPM has a rapid onset with a 6 up to 12-month delay in diagnosis research on biomarkers of early diagnosis should be driven to the discovery of more manageable tests. In conclusion, the up-to-date research suggests that, along with a more consolidated prognostic and monitoring role, some blood biomarkers especially when combined in specific panel are very promising also in the field of prevention and further research is highly recommendable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marsili D, Terracini B, Santana VS, et al. Prevention of Asbestos-Related Disease in Countries Currently Using Asbestos. Int J Environ Res Public Health 2016;13:E494. [Crossref] [PubMed]
- Hulka BS, Wilcosky TC, Griffith JD. editors. Biological markers in epidemiology. New York: Oxford University Press, 1990.
- American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004;170:691-715. [Crossref] [PubMed]
- Ollier M, Garcier JM, Naughton G, et al. CT scan procedure for lung cancer screening in asbestos-exposed workers. Chest 2014;146:e76-7. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015;41:5-15. [Crossref] [PubMed]
- Guglielmi G, Pantani E, Pistelli A, et al. Report of medical surveillance of workers exposed to asbestos at the Operative Unit of Occupational Medicine in Pisa. G Ital Med Lav Ergon 2012;34:574-6. [PubMed]
- Luo L, Shi HZ, Liang QL, et al. Diagnostic value of soluble mesothelin-related peptides for malignant mesothelioma: a meta-analysis. Respir Med 2010;104:149-56. [Crossref] [PubMed]
- Amati M, Tomasetti M, Scartozzi M, et al. Profiling tumor-associated markers for early detection of malignant mesothelioma: an epidemiologic study. Cancer Epidemiol Biomarkers Prev 2008;17:163-70. [Crossref] [PubMed]
- Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155-60. [Crossref] [PubMed]
- Rodríguez Portal JA, Rodríguez Becerra E, Rodríguez Rodríguez D, et al. Serum levels of soluble mesothelin-related peptides in malignant and nonmalignant asbestos-related pleural disease: relation with past asbestos exposure. Cancer Epidemiol Biomarkers Prev 2009;18:646-50. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Bonotti A, et al. Two novel polymorphisms in 5’ flanking region of the mesothelin gene are associated with soluble mesothelin-related peptide (SMRP) levels. Int J Biol Markers 2011;26:117-23. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Bonotti A, et al. Polymorphisms in the putative micro-RNA-binding sites of mesothelin gene are associated with serum levels of mesothelin-related protein. Occup Environ Med 2010;67:233-6. [Crossref] [PubMed]
- Hollevoet K, Nackaerts K, Thimpont J, et al. Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med 2010;181:620-5. [Crossref] [PubMed]
- Boudville N, Paul R, Robinson BW, et al. Mesothelin and kidney function--analysis of relationship and implications for mesothelioma screening. Lung Cancer 2011;73:320-4. [Crossref] [PubMed]
- Bonotti A, Simonini S, Pantani E, et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int J Biol Markers 2017;32:e126-31. [Crossref] [PubMed]
- Arnold DT, De Fonseka D, Hamilton FW, et al. Prognostication and monitoring of mesothelioma using biomarkers: a systematic review. Br J Cancer 2017;116:731-41. [Crossref] [PubMed]
- Bharadwaj U, Marin-Muller C, Li M, et al. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011;32:1013-24. [Crossref] [PubMed]
- Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 2006;5:50. [Crossref] [PubMed]
- Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem 2009;284:3739-49. [Crossref] [PubMed]
- Tang Z, Qian M, Ho M. The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents Med Chem 2013;13:276-80. [Crossref] [PubMed]
- Chang MC, Chen CA, Hsieh CY, et al. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem J 2009;424:449-58. [Crossref] [PubMed]
- Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 2001;7:3862-8. [PubMed]
- Coppola D, Szabo M, Boulware D, et al. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res 2004;10:184-90. [Crossref] [PubMed]
- Cristaudo A, Foddis R, Bonotti A, et al. Comparison between plasma and serum osteopontin levels: usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int J Biol Markers 2010;25:164-70. [PubMed]
- Rai AJ, Flores RM, Mathew A, et al. Soluble mesothelin related peptides (SMRP) and osteopontin as protein biomarkers for malignant mesothelioma: analytical validation of ELISA based assays and characterization at mRNA and protein levels. Clin Chem Lab Med 2010;48:271-8. [Crossref] [PubMed]
- Paleari L, Rotolo N, Imperatori A, et al. Osteopontin is not a specific marker in malignant pleural mesothelioma. Int J Biol Markers 2009;24:112-7. [Crossref] [PubMed]
- Hu ZD, Liu XF, Liu XC, et al. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: a systematic review and meta-analysis. Clin Chim Acta 2014;433:44-8. [Crossref] [PubMed]
- Shiomi K, Hagiwara Y, Sonoue K, et al. Sensitive and specific new enzyme-linked immunosorbent assay for N-ERC/mesothelin increases its potential as a useful serum tumor marker for mesothelioma. Clin Cancer Res 2008;14:1431-7. [Crossref] [PubMed]
- Sato T, Suzuki Y, Mori T, et al. Newly established ELISA for N-ERC/mesothelin improves diagnostic accuracy in patients with suspected pleural mesothelioma. Cancer Med 2014;3:1377-84. [Crossref] [PubMed]
- Onda M, Nagata S, Ho M, et al. Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res 2006;12:4225-31. [Crossref] [PubMed]
- Iwahori K, Osaki T, Serada S, et al. Megakaryocyte potentiating factor as a tumor marker of malignant pleural mesothelioma: evaluation in comparison with mesothelin. Lung Cancer 2008;62:45-54. [Crossref] [PubMed]
- Creaney J, Sneddon S, Dick IM, et al. Comparison of the diagnostic accuracy of the MSLN gene products, mesothelin and megakaryocyte potentiating factor, as biomarkers for mesothelioma in pleural effusions and serum. Dis Markers 2013;35:119-27. [Crossref] [PubMed]
- Creaney J, Yeoman D, Demelker Y, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol 2008;3:851-7. [Crossref] [PubMed]
- Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. [Crossref] [PubMed]
- Pei D, Li Y, Liu X, et al. Diagnostic and prognostic utilities of humoral fibulin-3 in malignant pleural mesothelioma: Evidence from a meta-analysis. Oncotarget 2017;8:13030-8. [Crossref] [PubMed]
- Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A 2010;107:12611-6. [Crossref] [PubMed]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1-5. [Crossref] [PubMed]
- Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res 2012;18:598-604. [Crossref] [PubMed]
- Wang Y, Faux SP, Hallden G, et al. Interleukin-1beta and tumour necrosis factor-alpha promote the transformation of human immortalised mesothelial cells by erionite. Int J Oncol 2004;25:173-8. [PubMed]
- Jube S, Rivera ZS, Bianchi ME, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res 2012;72:3290-301. [Crossref] [PubMed]
- Pellegrini L, Xue J, Larson D, et al. HMGB1 targeting by ethyl pyruvate suppresses malignant phenotype of human mesothelioma. Oncotarget 2017;8:22649-61. [Crossref] [PubMed]
- Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin Cancer Res 2016;22:3087-96. [Crossref] [PubMed]
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. [Crossref] [PubMed]
- Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5. [Crossref] [PubMed]
- Napolitano A, Pellegrini L, Dey A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016;35:1996-2002. [Crossref] [PubMed]
- Kittler R, Pelletier L, Heninger AK, et al. Genome-scale RNAi profiling of cell division in human tissue culture cells. Nat Cell Biol 2007;9:1401-12. [Crossref] [PubMed]
- Schlabach MR, Luo J, Solimini NL, et al. Cancer proliferation gene discovery through functional genomics. Science 2008;319:620-4. [Crossref] [PubMed]
- Kadariya Y, Cheung M, Xu J, et al. Bap1 Is a Bona Fide Tumor Suppressor: Genetic Evidence from Mouse Models Carrying Heterozygous Germline Bap1 Mutations. Cancer Res 2016;76:2836-44. [Crossref] [PubMed]
- Zauderer MG, Bott M, McMillan R, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 2013;8:1430-3. [Crossref] [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 2007;17:118-26. [Crossref] [PubMed]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 2006;11:441-50. [Crossref] [PubMed]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-14. [Crossref] [PubMed]
- Busacca S, Germano S, De Cecco L, et al. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol 2010;42:312-9. [Crossref] [PubMed]
- Tomasetti M, Staffolani S, Nocchi L, et al. Clinical significance of circulating miR-126 quantification in malignant mesothelioma patients. Clin Biochem 2012;45:575-81. [Crossref] [PubMed]
- Santarelli L, Strafella E, Staffolani S, et al. Association of MiR-126 with soluble mesothelin-related peptides, a marker for malignant mesothelioma. PLoS One 2011;6:e18232. [Crossref] [PubMed]
- Bononi I, Comar M, Puozzo A, et al. Circulating microRNAs found dysregulated in ex-exposed asbestos workers and pleural mesothelioma patients as potential new biomarkers. Oncotarget 2016;7:82700-11. [Crossref] [PubMed]
- Weber DG, Johnen G, Bryk O, et al. Identification of miRNA-103 in the cellular fraction of human peripheral blood as a potential biomarker for malignant mesothelioma--a pilot study. PLoS One 2012;7:e30221. [Crossref] [PubMed]
- De Santi C, Melaiu O, Bonotti A, et al. Deregulation of miRNAs in malignant pleural mesothelioma is associated with prognosis and suggests an alteration of cell metabolism. Sci Rep 2017;7:3140. [Crossref] [PubMed]
- Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6. [Crossref] [PubMed]
- Cristaudo A, Bonotti A, Simonini S, et al. Combined serum mesothelin and plasma osteopontin measurements in malignant pleural mesothelioma. J Thorac Oncol 2011;6:1587-93. [Crossref] [PubMed]
- Melaiu O, Cristaudo A, Melissari E, et al. A review of transcriptome studies combined with data mining reveals novel potential markers of malignant pleural mesothelioma. Mutat Res 2012;750:132-40. [Crossref] [PubMed]
- Giusti L, Da Valle Y, Bonotti A, et al. Comparative proteomic analysis of malignant pleural mesothelioma evidences an altered expression of nuclear lamin and filament-related proteins. Proteomics Clin Appl 2014;8:258-68. [Crossref] [PubMed]
- Bonotti A, Foddis R, Landi S, et al. A Novel Panel of Serum Biomarkers for MPM Diagnosis. Dis Markers 2017;2017:3510984.
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99.
- Creaney J, Robinson BW. Malignant Mesothelioma Biomarkers: From Discovery to Use in Clinical Practice for Diagnosis, Monitoring, Screening, and Treatment. Chest 2017;152:143-9. [Crossref] [PubMed]


