Feeding route or learning route for nutrition in critically ill
Feeding route
On 8 November 2017 the findings of the NUTRIREA-2 trial were published in the Lancet (1). The NUTRIREA-2 trial is a large (n=2,400) randomised controlled trial assessing the effect of the route of nutritional support in critically ill adults without contraindications to enteral nutrition (EN) or parenteral nutrition (PN). On 30 October 2014 the findings of the CALORIES trial were published in the New England Journal of Medicine (2) with the same comparison.
“NUTRIREA-2 focused on patients treated with invasive mechanical ventilation and vasopressor support for shock, because previous studies suggested that mechanically ventilated patients in ICU with haemodynamic instability might have better survival when early nutrition is given enterally rather than parenterally” (1). According to the authors of NUTRIREA-2 trial, nutritional intakes were far closer to targets than in the CALORIES trial, but this remains to be seen.
The outcome of NUTRIREA-2 is similar to CALORIES, the groups given early normocaloric enteral versus parenteral nutrition showed no significant differences in day 28 mortality and most other outcomes (including frequency of infectious complications). Gastrointestinal complications, including rare but severe complications, were however significantly increased in the EN versus PN group. This had not been observed in the CALORIES trial.
Learning route
While we now have two RCTs on the comparison of early EN with early PN with similar outcomes, we might learn that the general preference for EN is a bit misleading. There may not be a general preference, since it is essentially the individual patient that should be treated optimally either with EN or PN or both. The supposed higher level of infectious complications with PN, were not different between feeding routes, as also shown by CALORIES. Therefore these recent trials appear to indicate that in the UK and France this is not true (anymore). However, this new trial adds that EN can have severe gastrointestinal complications which have to be considered now as well. A recent meta-analysis suggested that complications might be more related to the dose of feeding than the route of feeding (3). The authors of NUTRIREA-2 claim “nutritional intakes were far closer to targets than in the CALORIES trial”. Since the dose of feeding appears to be relevant, let’s have a look.
Dose of feeding
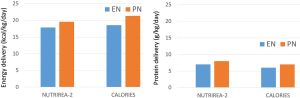
According to the authors of the two studies NUTRIREA-2 and CALORIES, there is no major difference in energy and protein delivery during early days after initiation of nutritional support, see Figure 1. In both studies the PN group is provided slightly more energy (~140–220 kcal/day and ~8 g protein/day; based on the similar BMI in both studies and using a body weight of 80 kg). One difference may be that in NUTRIREA-2 the caloric target is reached by day 1 and in CALORIES by day 3. However, the caloric target in NUTRIREA-2 was 20–25 kcal/kg/d and in CALORIES it was 25 kcal/kg/d. While in CALORIES only about 25% of patients reached their caloric target, this will be higher in NUTRIREA-2 simply on the basis of a lower caloric target. PN appears to be exclusive for 72 h (3 d) in NUTRIREA-2, but up to 5 days in CALORIES.
One size fits all?
The included patient populations are different between the two trials. Based on the SOFA score of 9.5 in the CALORIES and 11 in the NUTRIREA-2 trial, included patients were more severely ill in NUTRIREA-2 compared to CALORIES. The NUTRIREA-2 included shock patients, of which two third were septic shock patients. CALORIES included 16% of patients without mechanical ventilation. Therefore we have two trials on the comparison of early exclusive PN versus early exclusive EN, and 28- or 30-day mortality outcome is rather similar. The 90-day mortality appears to be worse in the more severely ill patients of the NUTRIREA-2 compared to CALORIES trial, again not different between PN and EN.
Based on current knowledge the early caloric delivery might be optimal at 70–90% of measured energy expenditure (4,5). In both NUTRIREA-2 and CALORIES trial only assumptions have been made on the individual caloric goal: 20–25 or 25 kcal/kg/day. This goal is of no relevance of interpretation of the trials (like number or percentage of patients not reaching goal). The mean value of caloric delivery is similar (~20 kcal/kg/day), and it can be assumed that a similar percentage of patients have had either caloric overfeeding or caloric underfeeding (6) in both trials. Since the NUTRIREA-2 trial appears to have fed patients more aggressively while in (septic) shock, a worse outcome would have been expected (7).
However, this comparison cannot be made as these are two RCTs in different settings. We can only compare the EN and PN arms of the trials, and in both trials it is observed that no differences in mortality are apparent. However, some more gastrointestinal problems appear in the EN versus PN group of the NUTRIREA-2 trial. This was not observed in the CALORIES trial. While the level of caloric delivery appears to be judged as playing a significant role, it may in fact be the somewhat earlier caloric delivery in the more severely ill patients that plays a role in the gastrointestinal problems that arise from this new trial.
Other trials have shown that in heterogeneous groups of critically ill patients, there can be a wide variety of caloric feeding without clear impact on mortality outcome (8). Both trials provide low levels of protein feeding at 0.6–0.8 g protein/kg/day, which is well below the recommended level of protein intake for critically ill patients (>1.2 g/kg/day). Again protein has not been studied, therefore, no conclusions can be drawn for protein delivery. New studies on the relevance of protein have to be designed (8).
The general conclusion, enteral nutrition is preferred in feeding critically ill, may now be softened to patient specific diagnosis and treatment goals which may either require enteral or parenteral nutrition or both. Less evidence-based and more context-based nutrition are in the light of personalized patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: Financial contributions have been received from Baxter, Fresenius, Nestle and Nutricia. All have been related to protein feeding, none were related to route of feeding.
References
- Reignier J, Boisramé-Helms J, Brisard L, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018;391:133-43. [Crossref] [PubMed]
- Harvey SE, Parrott F, Harrison DA, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014;371:1673-84. [Crossref] [PubMed]
- Elke G, van Zanten AR, Lemieux M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care 2016;20:117. [Crossref] [PubMed]
- Weijs PJ, Looijaard WG, Beishuizen A, et al. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 2014;18:701. [Crossref] [PubMed]
- Zusman O, Theilla M, Cohen J, et al. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016;20:367. [Crossref] [PubMed]
- Oshima T, Berger MM, De Waele E, et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr 2017;36:651-62. [Crossref] [PubMed]
- Reintam Blaser A, Starkopf J, Alhazzani W, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017;43:380-98. [Crossref] [PubMed]
- Arabi YM, Casaer MP, Chapman M, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med 2017;43:1239-56. [Crossref] [PubMed]