Diagnostic criteria for obstructive sleep apnea: time for reappraisal
The present diagnostic criteria for an obstructive sleep apnea syndrome (OSAS) were established by the Report of an American Academy of Sleep Medicine Task Force published in 1999 (1), and often referred to as the “Chicago criteria”. This report, co-authored by the present writer, specified that OSAS requires the presence of sleep disordered breathing (SDB) measured in an overnight sleep study combined with the presence of symptoms typical of the disorder, most notably excessive daytime sleepiness (EDS). Furthermore, different severity levels were identified according to the apnea-hypopnea frequency per hour (AHI) with an AHI >5 being required for significant SDB, an AHI between 5 and 15 representing mild, between 15 and 30 representing moderate, and AHI >30 representing a severe disorder when relevant clinical symptoms are also present. Based on these diagnostic criteria, the population prevalence of OSAS was estimated at 4% of adult males and 2% of adult females in the landmark Wisconsin cohort study published in 1993 (2).
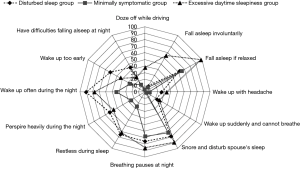
While these diagnostic criteria have served clinical sleep medicine reasonably well over the years, several limitations have become increasingly evident. Even prior to the introduction of the present diagnostic criteria, it was recognised that there is a poor correlation between symptom profile and the level of SDB based on AHI (3), and particularly so with EDS as measured by the Epworth Sleepiness Score (ESS) (4). A recent report from Iceland indicated a very high prevalence for SDB based on AHI but also very poor correlation of AHI with symptoms such as sleepiness (5). Nonetheless, effective control of SDB by nasal continuous positive airway pressure (CPAP) is typically associated with substantial improvements in symptom profile, both EDS (6) and also other related somatic symptoms (7). These apparently conflicting findings indicate that the assessment of OSAS severity should not be based on AHI alone and should take into account both the level of SDB and symptom profile, and possibly also related co-morbidities such as systemic hypertension, especially where associated with a nocturnal non-dipping blood pressure profile (8). This evolution in the approach to clinical diagnosis is underpinned by the recognition of different clinical phenotypes in OSAS (9-11), and furthermore that a range of pathophysiological phenotypes can also be identified (12). Co-morbidities such as hypertension appear to be more common in patients with minimal symptoms despite similar levels of SDB (10), and it is becoming increasingly recognised that symptoms relating to OSAS present in clusters that may significantly differ from each other in important aspects such as subjective sleep quality and level of daytime sleepiness (Figure 1) (10). Thus, these findings suggest that the comprehensive diagnosis of OSAS should be based on a blend of factors that include AHI, symptom profile such as ESS, and the presence of relevant co-morbidities such as non-dipping hypertension.
A further limitation of the present diagnostic classification of OSAS is the increasing recognition of a very high prevalence for the disorder. In particular, some of the most recent epidemiological data such as the HypnoLaus cohort study from Switzerland report prevalence figures of moderate or severe SDB up to 50% in adult males among the general population (5,13). On the other hand, these recent reports indicate that a significantly increased risk of co-morbidities is only evident in subjects with more severe SDB (13), and current evidence calls into question the clinical significance of mild sleep apnea as presently defined (14). Furthermore, the growing trend towards ambulatory diagnostic sleep studies that do not include sleep staging require a re-evaluation of the thresholds for mild, moderate, and severe categories as the AHI is generally underestimated in sleep studies that do not include sleep staging because of the measure being based on sleep period time rather than actual time spent asleep (15). Thus, an AHI within the mild range recorded in a sleep study based on cardiorespiratory polygraphy might rise into the moderate range in a polysomnography study after the exclusion of intervening periods spent awake during the study recording. A further factor complicating the diagnosis of OSAS, especially in the mild category, is the recognised night-to-night variability of SDB, which has been reported to be in the region of 30% in one recent report based on ambulatory studies (16).
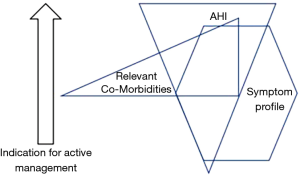
Although the formal diagnostic criteria for OSAS remain unchanged from those proposed in the Chicago criteria, clinical practice has evolved to take into account the high prevalence of SDB in the general population and the poor association daytime symptoms such as sleepiness. Thus, patients presenting with relatively mild OSAS based on AHI but who are very symptomatic may be given a trial of therapy with CPAP, typically for two months, and further management decisions are guided by the clinical response to this trial (14). In this context, the CPAP trial forms part of the diagnostic process rather than an active management decision. Furthermore, since the available evidence points to co-morbidities being influenced more by the level of SDB than by symptom profile such as sleepiness (9,10), the presence of co-morbidities such as non-dipping hypertension could be included in the assessment of disease severity, and particularly to decide on a threshold for initiating CPAP therapy. A proposed approach to the diagnosis of a clinically significant syndrome that prompts an active management approach is illustrated in Figure 2, which schematically highlights that AHI, symptom profile, and relevant co-morbidities such as hypertension may be present at different levels in an individual patient, and that a blend of these parameters can be used to guide the need for active management.
These considerations call into question the present disease severity classification based on the Chicago criteria and call for a new international consensus on the diagnosis and severity grading of this highly prevalent disorder. This call is reinforced by the recognition of OSAS as an important contributing factor in motor vehicle accidents and the recent introduction of Europe-wide regulations regarding fitness to drive for patients with the disorder (17). Thus, a personalised approach to the diagnosis and management of patients with OSAS is most appropriate (18), preferably by a clinician with experience and expertise in managing OSAS. This approach is most likely to achieve successful results in terms of patient satisfaction, optimum choice of therapy, and successful outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Flemons WW, Buysse D, Redline S, et al. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667-89. [Crossref] [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [Crossref] [PubMed]
- Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J 1996;9:117-24. [Crossref] [PubMed]
- Kingshott RN, Sime PJ, Engleman HM, et al. Self assessment of daytime sleepiness: patient versus partner. Thorax 1995;50:994-5. [Crossref] [PubMed]
- Arnardottir ES, Bjornsdottir E, Olafsdottir KA, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J 2016;47:194-202. [Crossref] [PubMed]
- Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 2006;61:430-4. [Crossref] [PubMed]
- Kiely JL, Murphy M, McNicholas WT. Subjective efficacy of nasal CPAP therapy in obstructive sleep apnoea syndrome: a prospective controlled study. Eur Respir J 1999;13:1086-90. [Crossref] [PubMed]
- Crinion SJ, Ryan S, McNicholas WT. Obstructive sleep apnoea as a cause of nocturnal nondipping blood pressure: recent evidence regarding clinical importance and underlying mechanisms. Eur Respir J 2017;49:1601818. [Crossref] [PubMed]
- Saaresranta T, Hedner J, Bonsignore MR, et al. Clinical phenotypes and comorbidity in european sleep apnoea patients. PLoS One 2016;11:e0163439. [Crossref] [PubMed]
- Ye L, Pien GW, Ratcliffe SJ, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J 2014;44:1600-7. [Crossref] [PubMed]
- Bailly S, Destors M, Grillet Y, et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One 2016;11:e0157318. [Crossref] [PubMed]
- Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188:996-1004. [Crossref] [PubMed]
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015;3:310-8. [Crossref] [PubMed]
- McNicholas WT, Bonsignore MR, Lévy P, et al. Mild obstructive sleep apnoea: clinical relevance and approaches to management. Lancet Respir Med 2016;4:826-34. [Crossref] [PubMed]
- Escourrou P, Grote L, Penzel T, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res 2015;24:730-8. [Crossref] [PubMed]
- Stöberl AS, Schwarz EI, Haile SR, et al. Night-to-night variability of obstructive sleep apnea. J Sleep Res 2017;26:782-8. [Crossref] [PubMed]
- Bonsignore MR, Randerath W, Riha R, et al. New rules on driver licensing for patients with obstructive sleep apnea: European Union Directive 2014/85/EU. J Sleep Res 2016;25:3-4. [Crossref] [PubMed]
- Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for obstructive sleep apnea. Chest 2017. [Epub ahead of print]. [Crossref]


