Stage I synchronous multiple primary non-small cell lung cancer: CT findings and the effect of TNM staging with the 7th and 8th editions on prognosis
Introduction
Multiple primary lung cancer (MPLC) is characterized by the simultaneous or successive occurrence of two or more primary lung cancers in the same individual. This condition can be classified as synchronous multiple primary lung cancer (SMPLC) and metachronous multiple primary lung cancer (MMPLC) according to the time of occurrence of foci. The diagnosis interval between 2 malignant lung tumors is often ≤6 months for SMPLC, although a different interval may be observed for MMPLC (1).
With the recent improvements in imaging technology, development of screening for high lung cancer risk, use of low-dose chest computed tomography (CT) screening, and physical examination, the detection rate of MPLC has been increasing annually. Studies have reported that up to 8% of non-small cell lung cancer (NSCLC) involve multiple lesions (1), particularly those of multiple primary pulmonary adenocarcinoma (2-5). In 2013, the American College of Chest Physicians (ACCP) issued new diagnostic criteria for MPLC (6), however, early diagnosis still needs to be combined with pathology, molecular biology, and imaging examinations, particularly for the differential diagnosis of metastatic tumors and granulomatous lesions. In recent years, the 5-year overall survival rate of SMPLC ranged from 34.0% to 60.9% (2,5,7-9). Some studies indicated that the prognosis of SMPLC is better than that of single primary lung cancer, except for the III A and IV stages (10). Nevertheless, to our knowledge, no detailed study has assessed the prognosis of early synchronous multiple primary non-small cell lung cancer (SMPNSCLC) (T1N0M0, T2aN0M0), or whether it is consistent with single primary lung cancer. In the present study, we aimed to assess the CT signs of 36 patients with stage I SMPNSCLC and compare the prognoses with those of patients with stage I solitary primary NSCLC (SPNSCLC). The new 8th edition of TNM staging for lung cancer is currently applied in the clinical setting (11,12). We also sought to compare the prognosis of SMPNSCLC with the 7th and 8th edition TNM staging systems (13), and thus determine a basis for the assessment of the early diagnosis and prognosis of lung cancer.
Methods
Subject
To perform this retrospective study, we obtained exemption and waivers from obtaining written informed consent from the institutional review board. We retrospectively examined the data of 1,948 patients with NSCLC who underwent surgery and pathological confirmation between January 1, 2009, and December 31, 2010, and underwent 5 years of follow-up (with complete data).
Diagnostic and staging criteria
Using the 2013 ACCP criteria and the 8th edition lung cancer stage classification (6,12), SMPLC was diagnosed as follows: the histological types are different, molecular genetic characteristics are different, or SMPLC originates from different in situ carcinoma; and the histology type is the same, but each lesion shows in situ carcinoma, tumors are present on different sides (leaves or segments of the lungs), no N1–N3 transfer is noted, and no systemic body transfer is observed.
In this study, 169 patients were enrolled based on the 7th edition of the lung cancer TNM staging system (13), and were then finally staged based on the maximum lesion diameter and the highest pathological stage. According to the 8th edition of the TNM staging system, if the maximum lesion diameter is >4 cm, it is classified as T2b, which represents non-early stage lung cancer (11,12). Thus, a total of 34 cases of stage I SMPNSCLC (excluding two cases), were detected and 111 cases of stage I SPNSCLC were detected (excluding 22 cases).
Research methods
All the cases were diagnosed by two senior radiologists and two senior pathologists. Stage I SMPNSCLC was confirmed by combining the clinical and imaging data, as well as the surgical histology, while excluding the lung metastases. The location (same lobes, different ipsilateral lobes, bilateral lobes), size, pathological type (adenocarcinoma, SCC, other), staging (Ia, Ib) and CT morphological features, including the following: lesion density [pure ground glass nodules (pGGNs), part-solid nodules (pSNs), solid nodules], shape (round/oval, irregular shape), edge (lobulation, spiculation), internal findings (bubble lucency, cavitation), and peripheral manifestations (bronchial truncation, pleural indentation) of each tumor were analyzed.
Follow-up was performed via telephone. The overall survival (OS) was estimated from the date of surgery to the day of death, whereas disease-free survival (DFS) was estimated from the date of surgery to the day of disease recurrence or death due to disease progression. The patients were followed up until December 31, 2015.
Kaplan-Meier single factor survival analysis was performed to assess the age, sex, smoking history, emphysema, and pathological type, as well as the tumor number, location, and size in the patients. Moreover, the survival prognosis of SPNSCLC was simultaneously examined. To controlled the factors of age, gender, smoking history, emphysema, pathological type, TNM stage, which might confound the interpretation of prognosis between SMPNSCLC and SPNSCLC, Cox regression analysis was performed. SPSS 22.0 was used for data processing. A P value of <0.05 indicates that the difference is statistically significant.
Results
Clinical, surgical, and pathological manifestations
The patients’ selection process is shown in Figure 1. Of these, 36 cases exhibited stage I SMPNSCLC (77 lesions; 1.85%), including 22 men and 14 women aged 44–86 years (median age, 60 years). Moreover, a total of 133 patients with stage I SPNSCLC were enrolled in this study, including 78 men and 55 women aged 19–85 years (median age, 61 years). Of 169 cases, 66 exhibited pulmonary lesions on physical examination, without any clinical symptoms. In the remaining 103 cases, clinical manifestations, including cough, chest pain, hemoptysis or sputum bloody, and shortness of breath, were first detected, followed by the observation of lung cancer on CT examination. All the patients underwent examination with brain magnetic resonance imaging, abdominal CT, and whole body bone scan (ECT). Eighteen patients underwent systemic PET/CT examination, excluding the hilar lymph nodes, mediastinal lymph node metastasis, and extrapulmonary metastasis. Surgical methods included standard lobectomy, localized resection, or sleeve resection, along with cleaning of the hilar and mediastinal lymph nodes.
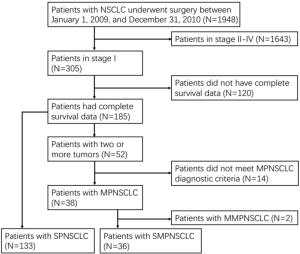
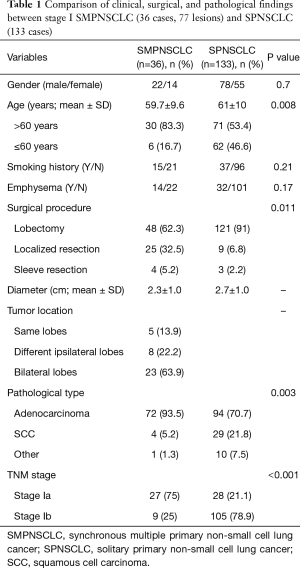
Based on the 7th edition, the comparison of the gender, age, smoking history, emphysema, surgical procedure, tumor size, location, type, and staging between stage I SMPNSCLC (36 cases) and stage I SPNSCLC (133 cases) is shown in Table 1.
Full table
Of the 36 cases of stage I SMPNSCLC, 33 (91.7%) had the same pathological type i.e., adenocarcinoma. In particular, 32 cases (88.9%) exhibited an adenocarcinoma–adenocarcinoma combination, including 14 cases with adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA). The combination of the specific pathological type and tumor location are detailed in Table 2.
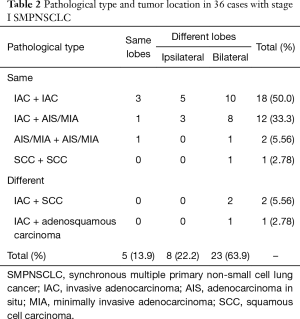
Full table
CT findings
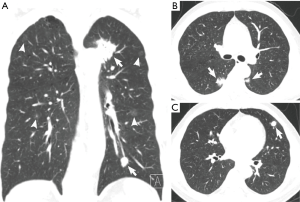
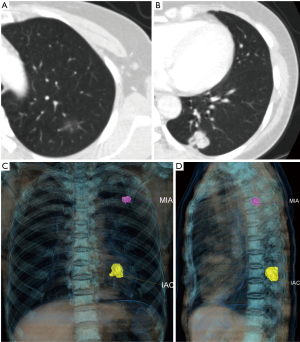
Based on the difference in lesion densities on CT, pulmonary nodules could be classified as solid and sub-solid nodules (including pGGNs and pSNs). The CT findings of all the lesions were analyzed, including the shape, edge, density, and internal and peripheral findings (Table 3,4). 7 cases with more separate solid lung cancers have different histologic type. CT primarily indicated a round/oval appearance, lobulation, and the spiculation sign, adjacent to the pleural indentation (Figure 2,3).
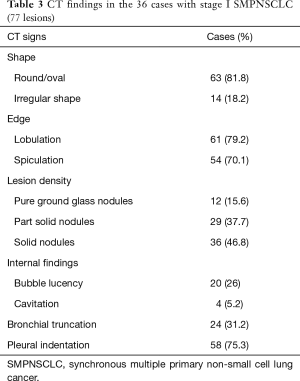
Full table
Full table
Prognosis
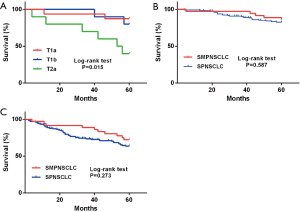
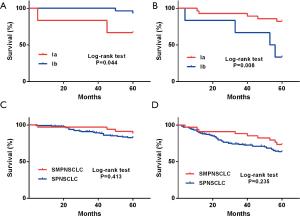
Based on 7th edition of the TNM staging system, the 5-year postoperative OS and DFS rates of stage I SMPNSCLC were 86.1% and 72.2%, respectively. Kaplan-Meier single-factor survival analysis was used to assess the age, sex, smoking history, emphysema, pathological type, and tumor number, location, and size among the patients. The findings showed that only the T stage was related to DFS; in particular, the proportion of patients with T1a, T1b, and T2a significantly differed according to the DFS (P=0.015; Figure 4A). However, when cases with stage I SPNSCLC were compared (5-year OS and DFS of 83.5% and 64.7%, respectively), the difference was not significant (P=0.587, P=0.273; Figure 4B,C).
Based on 8th edition of the TNM staging system, a total of 34 cases exhibited stage I SMPNSCLC in this group, and the 5-year OS and DFS rates were 88.2% and 73.5%, respectively. Kaplan-Meier single-factor survival analysis was used to assess the age, sex, smoking history, emphysema condition, T stage, operation pattern, pathological type, tumor number, and tumor location. The results indicated that the OS and DFS rates from stage Ia and stage Ib were significantly different (P=0.044, P=0.008; Figure 5A,B). However, when the 5-year OS and DFS rates of stage I SMPNSCLC were compared with those of stage I SPNSCLC (85.6% and 66.7%, respectively), the difference was not significant (P=0.413, P=0.235; Figure 5C,D).
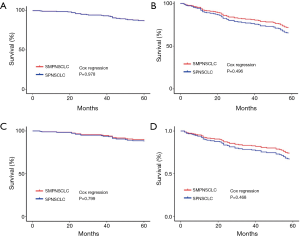
The Cox regression analysis showed that, based on 7th edition of the TNM staging system, the 5-year OS and DFS of stage I SMPNSCLC compared with those of stage I SPNSCLC, the difference was not significant (P=0.978, P=0496, respectively; Figure 6A,B); base on 8th edition of the TNM staging system, the difference was not also significant (P=0.799, P=0468, respectively; Figure 6C,D).
Discussion
The incidence of MPLC differs in various reports and ranges from 0.2% to 20%, most of which include cases of NSCLC. The detection rate of NSCLC has recently been increasing, and in the present study, we identified 36 cases of stage I SMPNSCLC, which accounted for 1.85% of the cases of NSCLC. Thus, the overall incidence of stage I SMPNSCLC is low, consistent with that reported in the literature (14).
Previous studies (2-5) showed that SMPNSCLC more commonly involves adenocarcinoma. The main SMPNSCLC pathological type in the present study was adenocarcinoma-adenocarcinoma (88.9%), consistent with the literature. The reason for this phenomenon may include the high current incidence of adenocarcinoma in NSCLC patients and the wide application of spiral CT examination, which markedly increased the detection rate of sub-solid nodules. In cases with solid tumor lesions, SMPNSCLC exhibited the characteristics of primary lung cancer; in particular, most were isolated or had an isolated round or oval nodular opacity, and exhibited lobulation or spiculation signs, as well as pleural indentation sign. Among the stage I SMPNSCLC cases, the spiculation sign was observed in 70.1% of patients, lobulation was noted in 79.2% of patients, and pleural indentation sign was observed in 75.3% of patients, consistent with that noted in the literature.
Previous studies suggested that the proportion of AIS and MIA in SMPNSCLC is high, which supports the trend of multiple centers of lung adenocarcinoma (15). Suzuki et al. found that up to 52% of cases of SMPLCs are associated with atypical adenomatous hyperplasia (16). In the present study, 41 (53.2%) lesions showed sub-nodules that included AIS or MIA, as confirmed by surgery and pathology examination, thus strongly suggesting that lung adenocarcinoma tends to occur in multiple centers. Hence, if lung cancer (mostly solid mass) is detected on CT, and if the other lobes or segments have ≥1 sub-solid nodules, SMPNSCLC should be considered, and if the side of the lungs or both lungs simultaneously exhibit multiple sub-solid nodules, SMPNSCLC should be initially considered.
Some patients with a solid primary lung cancer have one or more separate solid tumor nodule(s) of the same histologic type, whose staging is currently controversial. The three major lung cancer research institutions—the American Cancer Joint Commission (AJCC), International Union Against Cancer (UICC), and International Lung Cancer Research Association (IASLC)—have yet to reach a consensus regarding the staging of SMPLC.
As per the 8th edition of the lung cancer staging system, these tumors should be classified according to the location of the separate nodule relative to the index tumor—T3 for a same-lobe, T4 for a same-side (different lobe), and M1a for an other-side location—with a single N and M category (11,12). Previous studies indicated that, for patients without lymph node metastasis for SMPLC, treatment and prognosis should be performed according to the maximum diameter of the lesion or the highest stage (10). The prognosis is similar to that of single primary lung cancer at the same stage (17,18). Thus, the highest stage is an independent prognostic factor for SMPLC. Hence, each lesion should be assessed with TNM staging, and the largest lesion or the highest pathological stage should be considered as the final TNM stage, based on which the treatment can be designed.
According to the 7th and 8th edition of the TNM staging system for lung cancer, the prognosis of stage I SMPNSCLC in the present study did not significantly differ from that of stage I SPNSCLC. The reasons for this finding include: (I) the stage I SPNSCLC we screened had no lymph nodes and distant metastasis, and the prognosis was relatively good; and (II) adenocarcinoma was the main pathological type in stage I SMPNSCLC and had better prognosis, particularly AIS/MIA which exhibited lazy growth and had the best prognosis. A total of 72 patients (93.5%) with stage I SMPNSCLC exhibited adenocarcinoma in this group, including 14 cases (38.9%) with AIS/MIA. Due to the higher survival rate of patients, we believe that for stage I NSCLC (regardless of whether single or multiple primary cancer), radical surgery should be considered first when there is no mediastinal lymph node metastasis or systemic metastasis, and if heart and lung function and the general condition permits. The currently performed video-assisted thoracic surgery ensures minimal trauma and rapid postoperative recovery, and yields the same prognosis as traditional thoracotomy. For non-surgical resection of the lesions, the surgery can be integrated with chemotherapy, radiotherapy, stereotactic ablative body radiotherapy (SABR), radiofrequency ablation (RFA), and molecular targeted therapy. If this is not adopted, then the illness may be delayed, which could affect the prognosis of the patient.
The 8th edition of the TNM staging system for lung cancer was introduced in the clinical settings in January 2017 (11,12). The guideline subdivides T1 into T1a, T1b, and T1c, and T2 into T2a and T2b; hence, T2bN0M0 belongs to stage II, which belongs to stage I as per the 7th edition. Some studies have indicated that, when comparing the survival of cases of pT1-T4N0M0, the 8th edition of the TNM staging survival curve is better than that of the 7th edition (11). In the present study, 36 patients with stage I SMPNSCLC were examined via survival analysis with the 7th and 8th editions of the TNM staging system; the results indicated that there was no significant difference in the OS and DFS rates in the two staging systems.
There are some limitations of this study. First, patients with multiple pulmonary tumors who did not receive surgical intervention were not included in this retrospective study, which caused selection and information biases inherent. Additionally, the number of this study is small and included only stage I SMPNSCLC, we intend to study more cases, including stage II–IV patients in the future.
Conclusions
Adenocarcinoma was the main pathological type in stage I SMPNSCLC. Multiple synchronous lesions exhibited the malignant characteristics of primary lung cancer, particularly the presence of single or multiple sub-solid nodules. The prognosis of stage I SMPNSCLC was good, and did not show any significant difference with that of stage I SPNSCLC. The 7th and 8th edition of the TNM staging system for lung cancer yield consistent results during the postoperative evaluation of stage I SMPNSCLC patients.
Acknowledgements
Funding: The research was supported by Open Project of State Key Laboratory of Respiratory Disease (SKLRD2016OP011) and Science and Technology Planning Project of Guangdong Province (grant No. 2014A020212340 and 2017A040405065).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by our institutional review board, which waived informed consent.
References
- Battafarano RJ, Meyers BF, Guthrie TJ, et al. Surgical resection of multifocal non-small cell lung cancer is associated with prolonged survival. Ann Thorac Surg 2002;74:988-93; discussion 993-4. [Crossref] [PubMed]
- Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007;134:630-7. [Crossref] [PubMed]
- Mun M, Kohno T. Single-stage surgical treatment of synchronous bilateral multiple lung cancers. Ann Thorac Surg 2007;83:1146-51. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007;133:1193-200. [Crossref] [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg 2011;39:160-6. [Crossref] [PubMed]
- Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42. [Crossref] [PubMed]
- Fabian T, Bryant AS, Mouhlas AL, et al. Survival after resection of synchronous non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:547-53. [Crossref] [PubMed]
- Yu YC, Hsu PK, Yeh YC, et al. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg 2013;96:1966-74. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the t descriptors in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017;151:193-203.
- Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee. Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Arai J, Tsuchiya T, Oikawa M, et al. Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer 2012;77:281-7. [Crossref] [PubMed]
- Chen D, Mei L, Zhou Y, et al. A novel differential diagnostic model for multiple primary lung cancer: Differentially-expressed gene analysis of multiple primary lung cancer and intrapulmonary metastasis. Oncol Lett 2015;9:1081-8. [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastasis. Cancer 1997;79:1521-6. [Crossref] [PubMed]
- van Rens MT, Zanen P, Brutel de La Rivière A, et al. Survival in synchronous vs. single lung cancer: upstaging better reflects prognosis. Chest 2000;118:952-8. [Crossref] [PubMed]
- Walts AE, Mirocha JM, Leong T, et al. Pathologic staging and survival of patients with synchronous bilateral lung carcinomas. Am J Clin Pathol 2016;145:244-50. [Crossref] [PubMed]


