Expanding role for radiotherapy in metastatic non-small cell lung cancer in the era of targeted therapy and immuno-oncology
Radiotherapy for advanced stage non-small cell lung cancer (NSCLC) has traditionally been administered with palliative intent. However, an emergence of data from retrospective studies and, more recently, prospective trials has indicated the potential of using stereotactic ablative radiotherapy (SABR), in combination with known cytotoxic therapies, targeted therapies, and immunotherapeutic agents, to play a curative rather than palliative role in targeting sites of oligometastatic disease. Analysis of patterns of failure for oligometastatic NSCLC after first-line systemic therapy has revealed that failure occurs prevalently at sites of gross disease present at baseline rather than at new disease sites (1), and it has been postulated that local ablation of metastases could limit progenitor sites of new metastatic spread and extend progression free survival (PFS).
The oligometastatic state (Figure 1) has been recognized as an intermediate classification of advanced NSCLC in which disease has spread to a limited number of anatomic sites (2). Accordingly, these oligometastatic sites have been demonstrated to display clonal heterogeneity, inducing differential responses to systemic therapies (3). Traditional treatment paradigm has dictated that first-line systemic therapy is followed by maintenance therapy, and a new systemic therapy is initiated at the time of systemic failure, regardless of failure extent or magnitude. Local therapy, and specifically ablative radiotherapy, can exploit the inherent heterogeneity of oligoprogressive disease sites by eradicating site-limited disease failure which appears to have a different biology than system-wide progression. In a slightly different paradigm, radiotherapy in a consolidative setting can ablate remaining sites of disease after first-line systemic therapy, effectively eliminating resistant tumor deposits. Both approaches provide a way to extend the window of response and allow for the prevention of premature termination of a systemic therapy that resulted in site-limited failure. Collectively, these approaches point to consolidative radiotherapy as a means to achieving longer PFS, and potentially extend overall survival in the context of treating oligometastatic disease sites.
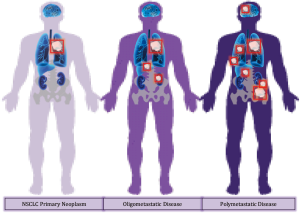
The multi-institutional phase II study conducted by the University of Texas MD Anderson Cancer Center, the London Health Sciences Center, and the University of Colorado addressed the prospective application of local consolidative therapy after first-line systemic therapy. Forty-nine patients with stage IV NSCLC were randomized 1:1 to receive either maintenance therapy alone or local consolidative therapy (radiotherapy, chemoradiotherapy or surgery) followed by maintenance therapy. The study revealed that PFS was significantly longer for the patient cohort receiving local consolidative therapy than for the patient cohort in the maintenance therapy arm of the trial: median PFS 11.93 vs. 3.9 months (HR =0.35; 90% CI: 0.18–0.66; P=0.0054), and 1-year PFS 48% vs. 20% (4).
In an analogous study, the single institution phase II trial conducted at the Harold C. Simmons Comprehensive Cancer Center at the University of Texas Southwestern Medical Center evaluated the benefit of consolidative radiotherapy in oligometastatic NSCLC. Twenty-nine patients were randomized 1:1 to either maintenance chemotherapy alone or SABR followed by maintenance chemotherapy. Paralleling the previous findings, median PFS was found to be 9.7 months for patients in the consolidative radiotherapy cohort, compared to 3.5 months for patients in the maintenance therapy arm (HR =0.304; 95% CI: 0.113–0.815; P=0.01). Of the 15 patients in the maintenance arm, 10 experienced progressions, with 7 of the 10 progression events occurring at original tumor sites; in the SABR arm of the study, only 4 out of 14 treated patients progressed, none within the radiated fields (5).
Both studies were stopped early to accrual after interim analysis determined local consolidative therapy to have a statistically significant, incontrovertible PFS benefit, which was defined as the primary study endpoint. PFS as the primary endpoint was advantageous both because of the early study termination and interim analysis, which precluded determination of the overall survival benefit, and the nature of PFS. PFS is not subject to the confounding effects of crossover and subsequent treatments, which complicated both studies, whereby patients in the maintenance therapy group received consolidative therapy. Three MD Anderson patients crossed over prior to progression (the PFS endpoint was not reached) and were censored at the time of crossover; two patients at the University of Texas crossed over to the SABR arm at oligoprogression.
While finding the survival advantage continues to prove difficult due to treatment crossover, scrupulous patient selection could aid in showing the survival advantage through vigilant follow-up and careful design. Patient selection criteria differed between the two studies in that the University of Texas study selected for patients with five or fewer sites of metastatic disease, while the MD Anderson study screened for patients with three or fewer metastatic disease lesions after first-line therapy. This difference emphasizes the importance of optimal patient selection criteria in terms of the extent of disease. Future trial design could benefit from carefully including patients with truly limited oligometastatic disease at presentation, as these patients have the highest potential to derive an overall survival benefit with consolidative local therapy to all sites of disease. Separation of patients with mutation positive NSCLC and patients with other potential prognostic factors could additionally reveal significant findings.
The treatment of patients with mutation positive NSCLC has progressively shifted towards molecular-guided therapies, and studies have shown a comparative advantage of tyrosine kinase inhibitors (TKIs) over standard chemotherapy. Yet while this shift towards targeted molecular therapy has heralded advances in disease-specific treatments, radiotherapy has remained in the background as a salvage therapy. Correspondingly, studies investigating the novel application of radiotherapy as a consolidative treatment have predominantly excluded EGFR and ALK mutation positive NSCLC, as did the University of Texas trial. The MD Anderson trial allowed for the enrollment of a wider range of patients and included patients having been treated with EGFR or ALK inhibitors. Although non-discriminatory for mutations largely because previous trials with more stringent patient selection criteria failed due to poor accrual, the MD Anderson study highlighted the use of radiotherapy beyond salvage therapy, particularly in NSCLC with EGFR or ALK mutations. While not analyzed separately because of the limited sample size, patients with mutation positive NSCLC accounted for 20% of the cohort receiving local consolidative therapy. The survival benefit of consolidative radiotherapy for this patient subset requires further corroboration, but these early results suggest the prospective application of radiotherapy beyond its limited use as salvage therapy.
As molecular-guided therapies have gained attention for mutation positive disease, efforts have increased to evaluate the role of radiotherapy at the forefront of treatment for mutation positive NSCLC. Currently, the London Institute of Cancer research is conducting the HALT trial in an effort to discern the benefit of radiotherapy ablation of oligoprogressive disease sites in prolonging response to TKI therapy, with patient selection criteria requiring confirmed treatment response to TKI therapy and three or fewer sites of oligoprogressive disease after inevitable therapy resistance (6) with a primary endpoint of PFS.
A fundamental difficulty of TKI therapy is the eventual development of acquired resistance. Approximately 60% of patients acquire the EGFR T790M resistance mutation, and this key clinical subset of patients has been successfully targeted by the third-generation TKI osimertinib (7). The AURA trial investigated the application of osimertinib as a first-line therapy in EGFR-mutated NSCLC patients, revealing improved PFS and suggesting a benefit to treating patients with this third-generation TKI upfront rather than after progression (8). Osimertinib as a front-line therapy may serve to increase the window of treatment response to TKI therapy—combined with radiotherapy, this treatment could allow for an even greater extension of response, with radiotherapy serving to ablate TKI-resistant disease sites. Future trials are necessary to investigate this prospective cooperative effect with more effective third-generation TKI such as osimertinib. While no single solution exists, in limited oligometastatic NSCLC, TKI therapy followed by consolidative radiotherapy or radiotherapy to treat oligoprogressive disease, as well as initial radiotherapy followed by TKIs, should be considered.
Disease that has progressed on immunotherapy—in the same manner as with chemotherapy—is also treated with salvage radiotherapy. A second treatment setting elucidated here is consolidative, whereby radiotherapy would target sites of persistent disease before immunotherapeutic failure. Using SABR as a consolidative therapy before progression might improve overall survival, and would again allow for the continuation of immunotherapeutic drugs for a longer duration. Radiotherapy has been noted to boost the immune response and sensitize patients to immunotherapeutic agents, and it could serve to improve survival synergistically with immunotherapy through both local and abscopal effects (9). The recent PACIFIC trial—a prospective study evaluating consolidative therapy with the PD-L1 inhibitor durvalumab after chemoradiotherapy for locally advanced NSCLC (10)—evidenced the success of definitive chemoradiotherapy treatment of sites of initially bulky disease followed by immunotherapy. Similar success may be attainable for oligometastatic NSCLC treated with this combination, and future trials are indicated to determine whether such treatment will confer the survival advantage of the previously shown synergy between immunotherapy and radiotherapy.
Numerous trials have suggested the efficacy of radiotherapy in combination with immunotherapy, chemotherapy, and TKIs in prolonging PFS; however, few have demonstrated a clear overall survival benefit. As more data accumulates in support of the use of local consolidative therapy, the challenge remains to further substantiate these preliminary findings with clinical trials designed to determine overall survival benefit which can be diluted by cross-over of the patients from the control arm to the experimental arm. This again emphasized the importance of novel trial design and careful patient selection to unequivocally prove the value of radiotherapy in the era of targeted therapy and immune-oncology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rusthoven KE, Hammerman SF, Kavanagh BD, et al. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol 2009;48:578-83. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget 2015;6:8491-524. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small- Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- McDonald F, Guckenberger M, Popat S, et al. HALT: targeted therapy beyond progression with or without dose-intensified radiotherapy in oligoprogressive disease in oncogene addicted lung tumours. Lung Cancer 2017;103:S57. [Crossref]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]


