Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study
Introduction
With the constantly increasing number of chest computed tomography (CT) scans performed each year, more and more lung lesions are being diagnosed (1); such as ground glass nodules, which can often be pre-invasive lesions [with no solid radiologic criteria to confidently distinguish benign lesions from malignant lesions (2)] and early stage lung cancer.
The American College of Chest Physicians recommends sublobar resection, preferably an anatomic segmentectomy, for patients with clinical stage I and II non-small cell lung cancer (NSCLC) who may tolerate surgery but not lobar resection. Sublobar resection (segmentectomy or wedge resection) is recommended for patients with stage I ground glass opacity ≤2 cm (3). The long-term oncologic efficiency of segmentectomy versus lobectomy for small NSCLC has yet to be demonstrated (4).
Segmentectomy has technical and anatomical limitations. Robotic surgery [robotic-assisted thoracic surgery (RATS)] and three-dimensional (3D) reconstruction should overcome these limitations.
Because of anatomical variations (especially in the courses of pulmonary arteries), perfect anatomical knowledge of the patient is required to minimize complications and optimize success of segmentectomy (especially for upper and basal segments). A standard chest CT scan might be sufficient, but the use of a 3D model allows full operative planning (5), from port placement (6) to anatomical resection (7,8).
Although 3D reconstruction can be achieved with software such as Advantage Workstation Volume Share (GE Healthcare™, Milwaukee, WI, USA) (9) or Synapse Vincent (Fujifilm™ Co., Tokyo, Japan) (5,8), this is time consuming and relies heavily on medical resources. Thus, we chose to outsource to Visible Patient™ (Strasbourg, France), a company specialized in this area.
To be usable in daily practice, we considered that several requirements should be respected, as shown in Table 1.
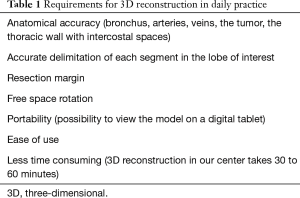
Full table
The objective of our pilot study was to assess if 3D reconstruction performed by Visible Patient™ could meet all these criteria and could be helpful for the operative planning, efficiency, and safety of mini-invasive thoracic surgery.
Methods
The present study was approved by the Review Board of our institution (No. E2017-31) and written consent was obtained from each patient to send the anonymized data from their CT to Visible Patient.
Inclusion and exclusion criteria
Inclusion criteria: patients with a progressing ground glass nodule ≤2 cm, compromised patients with stage I or II solid tumors, patients with small sized solid tumors with no histological documentation (negative CT-guided and fibroscopic biopsy), defined as not resectable by wedge resection by the treating surgeon.
Exclusion criteria: patients with lesions extending beyond a single lung segment, rapidly progressive or profuse metastatic diseases, and patients unfit for general anaesthesia.
Pre-operative assessment
Each patient was assessed by CT (thorax, abdomen, and brain), PET-CT, respiratory function evaluation, cardiologic assessment (ECG and sometimes echocardiography), and bronchoscopy.
Image acquisition and 3D reconstruction
All patients had chest CT performed on a 64-CT scanner (Optima CT540, GE Healthcare™, Milwaukee, WI, USA). A total volume of 80 mL non-ionic iodine-based contrast (Omnipaque 350, GE healthcare™, Milwaukee, WI, USA) was administered at a rate of 3.5 mL/sec. The scanning delay time was determined using bolus-tracking software to ensure full opacification of pulmonary arteries, pulmonary veins and aorta. The raw data were reconstructed at 0.6 mm thick in both parenchymal and mediastinal filter and window settings. All images were anonymized and the Digital Imaging and Communications in Medicine (DICOM) data were sent electronically for 3D reconstruction.
3D reconstructions were performed by a highly specialized private company (Visible Patient™, Strasbourg, France), using their own software. Time and cost of reconstruction depended on the complexity ordered. For instance, the delivery time was longer and the cost was higher for reconstruction of the entire chest wall as opposed to reconstruction of the bronchi of a single lobe. Reconstruction was performed by radiological technicians, who contoured every anatomical structure in the CT, slice by slice, to create the 3D image. Each lobar and segmental bronchus was manually labelled by the technician, lobar and sublobar segmentation was then automatically performed by the software.
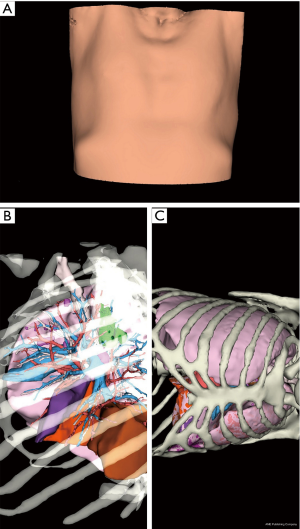
After a few days (generally 1 to 2 weeks), images were sent back to the surgical team and reviewed before and during surgical procedure with a dedicated application (VP Planning) on a digital tablet (iPad, Apple™, Cupertino, CA, USA) (Figure 1) or a computer.

The tablet was placed next to the robot console and its image was fed to the surgical screen (Figure 2).

Surgical procedure
All procedures were performed under general anaesthesia, using the Da Vinci® robot (Intuitive Surgical, Sunnyvale, CA, USA).
We used a modified total port-access video robot-assisted three arms technique, described by Ninan and Dylewski (12).
Results
Between 2014 and 2015, 9 segmentectomies (1 right S1, 2 basal segmentectomies, 1 left S1+2, 3 right and left S6, 1 right S2 and 1 left S4+5) were performed with a pre-operative 3D model (examples are given in Figures 3-5).
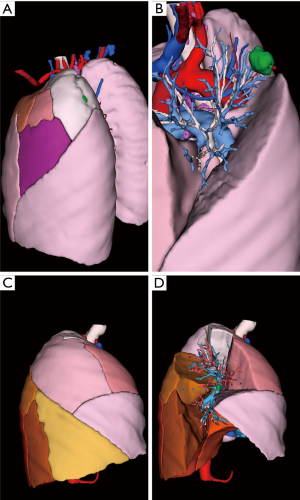
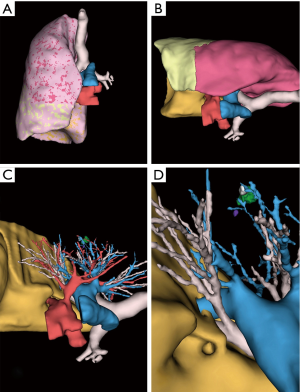
All 3D reconstructions met our expectations (Table 1) and were used successfully before and during surgery. We found no difference between the anatomy displayed in reconstructions and surgical findings.
To obtain the 3D reconstruction the surgeon uploaded the anonymized DICOM files to the secured Visible Patient™ server and then downloaded the model in the viewer (VP Planning application) after completion.
The viewer application also shows the volume of each lobes and segments.
3D model rotation allowed optimal positioning of the patient, as well as optimal port placement: the shape of the thorax being variable (long, wide), the best intercostal space is selected thanks to the 3D reconstructions with the ribcage in transparency, to clearly visualize the target and its relations with the intercostal spaces (Figures 2,5).
Putting the tablet in the robot console allowed live comparison of anatomy between the surgical view and the 3D model (Figure 2).
Discussion
3D CT reconstruction offers many advantages, such as increasing the surgeon’s confidence with effective operative planning, from port placement to resection. Visualization of the 3D model during the procedure allows full anatomical understanding (especially anatomical variations) and improves the surgery with better patient and port positioning, thus potentially improving surgical safety. We also added imaging to our surgical safety checklist (all anatomical variations were announced out loud before the procedure).
More particularly, Visible Patient™ 3D models were of good quality and met all our requirements (Table 1). Also, one of the main features of these reconstructions is the possibility to view them on portable supports, which is very convenient, as these latter can be carried and then placed in the intuitive robot console. The fact that 3D reconstruction is performed by a private company is also less time consuming. Although 3D reconstruction is performed at our tertiary care center by radiologists specialized in using Advantage Workstation Volume Share (GE Healthcare™, Milwaukee, WI, USA) (9), the process can take up to 1 hour. This is a significant waste of time because it is done within a full CT schedule, disrupting the radiologist’s workflow. In addition, the final product does not offer the same features as Visible Patient™ such as sublobar segmentation, the possibility to show/hide all anatomical structures, not to mention portability (it is possible to save and send selected DICOM images but not the 3D model as a whole).
The viewer application also shows the volume of each lobe and segment, which is variable from one patient to another, thus enabling informed choice of the best surgical technique between sublobar and lobar resection. For instance, standard lobectomy might be preferred to basal segmentectomy if the volume of the upper (S6) segment is negligible.
Nevertheless, 3D CT reconstruction has several disadvantages; mainly its high cost. In addition, it must be ordered ahead of time as delivery can take up to 2 weeks and the DICOM data has to be carefully anonymized. More generally, 3D CT reconstruction requires optimal quality CT (complete vascular opacification of systemic and pulmonary arteries and veins, good inspiration and no movement or respiratory artefacts).
A future challenge for robotic surgery is the implementation of augmented reality, when the 3D model will be successfully merged live with the thoracoscopic image, allowing a view of the tumor, bronchus and vessels through the lung. This has already been done, but mostly in hepatic surgery (13). The main difficulty in thoracic surgery is that the lung does not have the same anatomical configuration during surgery and on CT, due to deflation and pneumothorax. For unpalpable tumor localizations only, this limitation has successfully been bypassed with the use of peri-operative cone-beam CT (14).
Conclusions
In conclusion, we have shown that 3D CT operative planning using Visible Patient™ reconstruction is useful in our practice of robot-assisted segmentectomy. Its impact on reducing complications and improving surgical efficiency is the object of an ongoing study.
Acknowledgements
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript. Visible Patient™ graciously provided Rouen University Hospital with 15 free 3D reconstructions. This work was part of an ERDF (European Regional Development Fund) tender for digital patient development.
Footnote
Conflicts of Interest: JM Baste discloses fees for lecturing and proctoring for Intuitive Surgical. This work was presented at the French Thoracic and Cardio-Vascular Society autumn 2017 meeting.
Ethical Statement: The present study was approved by the Review Board of our institution (No. E2017-31) and written consent was obtained from each patient to send the anonymized data from their CT to Visible Patient.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: Diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:7S–37S.
- Linden D, Linden K, Oparka J. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy a suitable alternative to thoracotomy and segmentectomy in terms of morbidity and equivalence of resection? Interact Cardiovasc Thorac Surg 2014;19:107-10. [Crossref] [PubMed]
- Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-301. [PubMed]
- Wen J, Hou X, Chu X, et al. Application of three dimensional reconstruction technique in selection of incision of thoracic surgical operation with robot. Int J Clin Exp Med 2015;8:17818-23. [PubMed]
- Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Ikeda N, Yoshimura A, Hagiwara M, et al. Three Dimensional Computed Tomography Lung Modeling is Useful in Simulation and Navigation of Lung Cancer Surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. [Crossref] [PubMed]
- Matsumoto T, Kanzaki M, Amiki M, et al. Comparison of three software programs for three-dimensional graphic imaging as contrasted with operative findings. Eur J Cardiothorac Surg 2012;41:1098-103. [Crossref] [PubMed]
- Le Moal J, Peillon C, Dacher JN, et al. Visualization of 3D reconstruction on a smartphone. Asvide 2017;4:049. Available online: http://www.asvide.com/article/view/22647
- Le Moal J, Peillon C, Dacher JN, et al. Pre and intraoperative use of 3D reconstruction. Asvide 2017;4:050. Available online: http://www.asvide.com/article/view/22650
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Ntourakis D, Memeo R, Soler L, et al. Augmented Reality Guidance for the Resection of Missing Colorectal Liver Metastases: An Initial Experience. World J Surg 2016;40:419-26. [Crossref] [PubMed]
- Rouzé S, de Latour B, Flécher E, et al. Small pulmonary nodule localization with cone beam computed tomography during video-assisted thoracic surgery: a feasibility study. Interact Cardiovasc Thorac Surg 2016;22:705-11. [Crossref] [PubMed]


