Multiple intracranial aneurysms followed left atrial myxoma: case report and literature review
Introduction
Cardiac myxoma is the most common primary benign tumor of mesenchymal origin in the heart. More than 80% of this tumor are found in the left atrium (1,2). Patients with cardiac myxomas usually present a variety of neurologic syndromes even in the absence of cardiac symptoms (3). Systemic embolus is the second frequent presentation (29%) in patients with left cardiac myxomas. The emboli are most frequently related to cerebral vessels with stroke (21%) (4). The mobility, but not the size of the myxomas may be related to embolic potential (3).
Although cardiac myxoma-related cerebral embolus is well documented, there are also some other rare neurological complications such as cerebral aneurysms (5-7), myxomatous metastasis (3,7,8), and cerebral cavernous malformations (9). Here we describe a patient with multiple cerebral aneurysms after resection of cardiac myxoma and review the previous literature to get some primary conclusions about the clinical features, etiopathogenisis, diagnosis and therapy of the disease.
Case report
A 46-year-old woman was admitted to our hospital with a sudden anaesthesia of the right upper limb. Moreover, she had complained of paroxysmal and progressive headache for three months. Medical history revealed that she had a history of cardiac myxoma excision three years before and suffered hypertension for recent two years. She took oral anti-hypertension medicines regularly and the blood pressure was kept in normal range.
During her last hospitalization three years earlier, she presented with chest distress and breathlessness for two months without any neurologic manifestation. Physical examination suggested a Grade III apical diastolic murmur and neurologic examination was normal. Doppler echocardiography showed a large parenchymatous mass with clear boundary, diameter 4.7 cm × 5.5 cm × 3.1 cm, which originated from inferior inter-atrial septum and prolapsed into the left ventricle through mitral valve during diastole. The ejection fraction was 60.5%, mild mitral and tricuspid regurgitation were also found. The pulmonary arterial systolic pressure was evaluated at 52 mmHg, which suggested pulmonary hypertension. The patient underwent a thoracotomy, with resection of the myxoma. Immediately after the operation the patient reported marked relief of her chest distress and breathlessness. The control echocardiographic didn’t find pulmonary hypertension. The follow-up of the patient 53 months after the operation was totally asymptomatic with no evidence of recurrence at the echocardiographic examination.
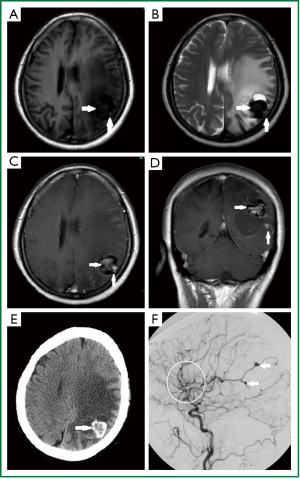
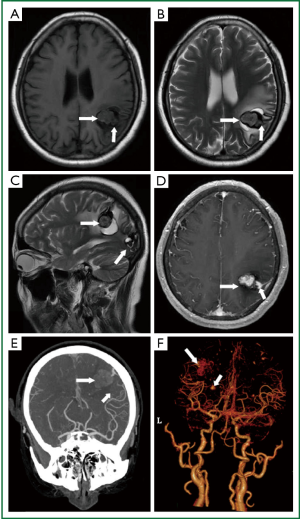
During the present hospitalization, she complained of no cardiac syndrome. Neurological examination was normal. The laboratory tests such as erythrocyte sedimentation rate, antistreptolysin O and C reactive protein were normal. Brain MRI (Figure 1A-D) demonstrated multiple abnormal signal intensity in cerebral cortex. Some lesions were mixed hypo- and hyper- intensity on both T1- and T2-weighted imaging. They could be contrasted enhanced on T1-weighted imaging. Based on the profile of the lesions and the surrounding liquefaction, we inferred that some lesions were areas of hemorrhage and the others may be aneurysms. CT scan (Figure 1E) showed a low density lesion surrounded by high density in the parietal lobe with severe edema and an inhomogeneous high-density lesion suspected of calcification in the left occipital lobe and right frontal lobe. DSA (Figure 1F) was recommended to further identify the lesions. Results demonstrated multiple fusiform cerebral aneurysms mostly on the middle and some on anterior artery. The largest two aneurysms were located on the distal branches of left middle cerebral artery. These areas were consistent with the result of MRI. We further confirmed that the larger lesion showed on MRI is hemorrhage area caused by rupture of the aneurysms. Mannitol was used to decrease the intracranial pressure and other symptomatic treatments were performed. Surgical resection was recommended for the patient because of the obvious mass effect with marked shift of middle line. Chemotherapy or radiation therapy were also suggested, the patient refused the above treatments. She was discharged after her headache and anesthesia was released. Follow-up of the patient showed no aggravating clinical manifestation and she received computed tomography angiography (CTA) instead of the DSA examination. The latest examination showed stable appearances of the aneurysms, and no sign of newly discovered aneurysms was found (Figure 2).
Discussion
Cerebral aneurysm followed atrial myxoma as a neurologic complication is rare. About 50 cases have been reported since the first reported case in1966 (10).
These aneurysms are usually multiple with fusiform or saccular shape, but mostly fusiform (91%) (5). They could appear alone or in combination (5,11-14). In the present case, fusiform and saccular aneurysms were both observed. Most of the aneurysms are located in distal branches of both sides of middle cerebral artery (5). Sabolek et al. (5) found that about 56% (19/34) of the aneurysms were found prior to tumor resection while the rest were detected after mass resection. The latency between tumor resection and aneurysm detection varies between 2 and 300 months (median: 36.0 months) (5). We conclude that the present patient’s cerebral aneurysms is a late complication of cardiac myxoma mainly because no other risk factors were found and occurrence of the patient’s cerebral aneurysms after cardiac myxoma excision is within the window of time mentioned above.
Mechanism of the disease has not been well understood. The theory of “Metastasize and Infiltrate” is mostly recognized. Myxomas fragments can metastasize to the brain. Then the myxomatous cells may infiltrate the vessel walls via mitotic and proliferating activity within the vessel and interrupt the elastic lamina (3,4,15,16), leading to vessel dilatation and aneurysms formation. Yaguchi et al. (17) reported a patient with new-formed cerebral aneurysms after resection of cardiac myxoma. No embolism or recurrence of cardiac myxoma was found. The level of IL-6 produced by myxoma cells was high in CSF while normal in serum. This case provided a good evidence to support the theory. Histopathologic evidence is further demonstrated by Rodriguez et al. (8). Interleukin-6 produced by myxoma cells may play an important role in the formation of cerebral aneurysms. High level of IL-6 produced by cardiac myxomatous cells in CSF has been reported (17). But most of the cases have no data about the IL-6 level in CSF (18). Whether IL-6 in CSF is elevated and whether it is produced by myxomatous cells in the intracranial vessels need to be further studied. The role of IL-6 is also supported by Koo et al. (11), who suggested that IL-6 can promote myxoma invasion into the cerebral arteries. IL-6 may promote invasion of myxomatous cells via matrix metalloproteinases (MMPs) because IL-6 can up-regulate the expression and activity of MMPs (19), which can degrade the extracellular matrix.
Patients with multiple cerebral aneurysms should prospectively receive echocardiography for myxoma (18). CT, MRI and cerebral angiography are elective methods in diagnosis of myxomatous cerebral aneurysms.
On MRI, the lesions may be characterized by tubular dilatations of arteries within the sulci and some hemorrhagic changes on T1-and T2-weighted imaging (5,20,21). In some cases the sulci may be hyperintense rims on T1-weighted imaging while on gradient-echo susceptibility imaging it is a marked blooming pattern of signal loss resulting from combined effects of either the dense deposits of hemosiderin and iron breakdown products (21). Moreover, the lesions appeared as contrast enhancing focal dilatations of distal segments of intracranial arteries on T1-weighted images. That may be a result of slow flowing inside the aneurysms or possibly enhanced myxoma tissue within the aneurysm wall. On T2-weighted images, they are characterized by low signal intensity flow voids, sometimes associated with cerebral infarctions (22). Magnetic resonance angiography (MRA) may confirm the nature of these lesions (21).
Myxoma-related cerebral aneurysms may appear as hyper-density on CT (23,24). In some cases, they look like calcification. Hwang et al. (21) found no evidence of calcification but the presence of myxoid matrix, iron, hemosiderin and red blood cells. That may explain the hyper-density lesion in most reported cases including the present patient. CTA in a high resolution CT scanner is necessary when MRA is likely insufficient to rule out a myxomatous aneurysm (25).
Cerebral DSA may be a better choice comparing with CT or MRI. DSA is free from the disturbance of edema or hemorrhage, demonstrating the dilated vessel and peripheral distribution of the cerebral arteries clearly. Cerebral aneurysms can appear as irregular fusiform outpouchings or saccular dilatations on angiography (22). The greatest part of aneurysms are localized on middle cerebral artery (74.2%) with slight laterality toward the right side, then anterior cerebral artery (13%), cerebellar arteries (7%), posterior cerebral artery (5%) and the least is basilar artery (1%) (5). Since small peripheral aneurysms, which are more frequent, can only be detected on DSA by delayed washout of contrast relative to the arterial phase, a finding that cannot be appreciated on MRA or CTA, DSA may be the most sensitive imaging method in diagnosis of small peripheral aneurysms (26).
No definite guidelines for therapy of the disease have been formulated. Resection of the cardiac myxoma is useful in eliminating early neurologic symptoms (11,13,27,28), but cannot completely abolish the risk of delayed cerebral aneurysm formation (5,12,17). This can be explained by the “Metastasize and Infiltrate” theory mentioned above. Chemotherapy results were equivocal (29). Radiation therapy in combination with chemotherapy has been reported as an effective way in degradation of myxoma metastasis (30), therefore it may be useful in preventing the myxomatous aneurysms. But we don’t find any related reports. Coil embolization for multiple cerebral aneurysms secondary to cardiac myxoma is still limited, mainly because most of the aneurysms are fusiform without a neck and cannot be clipped or coiled (24). Nevertheless there is still one report about successful coil embolization on a patient with giant neoplastic aneurysms from myxoma (31). Surgical treatment is controversial. The aneurysms located in the non-functional area such as the frontal lobe can be removed by surgical resection of the brain tissue (8), but not suitable for the functional area. If the lesions caused obvious mass effect, surgical resection is recommended to avoid the occurrence of cerebral hernia. The treatments need to be further studied.
Patients with multiple cerebral aneurysms, especially young patients, should be alerted to cardiac myxoma or the resection history. Similarly, patients with cardiac myxoma should be observed for myxoma-related cerebral aneurysms as well as the cerebral embolism and the tumor must be resected as soon as possible to prevent further complications. Long-term regular follow-up of these patients is recommended.
Acknowledgements
This study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Disclosure: The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Livi U, Bortolotti U, Milano A, et al. Cardiac myxomas: results of 14 years’ experience. Thorac Cardiovasc Surg 1984;32:143-7. [PubMed]
- Bakkali A, Sedrati M, Cheikhaoui Y, et al. Cardiac myxomas (a series of 23 cases). Ann Cardiol Angeiol (Paris) 2009;58:94-8. [PubMed]
- Lee VH, Connolly HM, Brown RD Jr. Central nervous system manifestations of cardiac myxoma. Arch Neurol 2007;64:1115-20. [PubMed]
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159-72. [PubMed]
- Sabolek M, Bachus-Banaschak K, Bachus R, et al. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: a case report and review. Acta Neurol Scand 2005;111:345-50. [PubMed]
- Messouak M, Zaam A, Maaroufi M, et al. Cardiac myxoma complicated with cerebral aneurysms and revealed by an ischemic stroke. Rev Neurol (Paris) 2011;167:150-4. [PubMed]
- Radoi MP, Stefanescu F, Arsene D. Brain metastases and multiple cerebral aneurysms from cardiac myxoma: case report and review of the literature. Br J Neurosurg 2012;26:893-5. [PubMed]
- Rodriguez FJ, Brown RD, Mohr JP, et al. Embolic atrial myxoma with neoplastic aneurysm formation and haemorrhage: a diagnostic challenge. Neuropathol Appl Neurobiol 2006;32:213-6. [PubMed]
- Sharma S, Tsyvine D, Maldjian PD, et al. An intriguing co-existence: atrial myxoma and cerebral cavernous malformations: case report and review of literature. J Am Soc Echocardiogr 2011;24:110.e1-4.
- Stoane L, Allen JH Jr, Collins HA. Radiologic observations in cerebral embolization from left heart myxomas. Radiology 1966;87:262-6. [PubMed]
- Koo YH, Kim TG, Kim OJ, et al. Multiple fusiform cerebral aneurysms and highly elevated serum interleukin-6 in cardiac myxoma. J Korean Neurosurg Soc 2009;45:394-6. [PubMed]
- Jean WC, Walski-Easton SM, Nussbaum ES. Multiple intracranial aneurysms as delayed complications of an atrial myxoma: case report. Neurosurgery 2001;49:200-2; discussion 202-3. [PubMed]
- Li Q, Shang H, Zhou D, et al. Repeated embolism and multiple aneurysms: central nervous system manifestations of cardiac myxoma. Eur J Neurol 2008;15:e112-3. [PubMed]
- Santillan A, Sigounas D, Fink ME, et al. Multiple fusiform intracranial aneurysms 14 years after atrial myxoma resection. Arch Neurol 2012;69:1204-5. [PubMed]
- Furuya K, Sasaki T, Yoshimoto Y, et al. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. Case report. J Neurosurg 1995;83:170-3. [PubMed]
- Burton C, Johnston J. Multiple cerebral aneurysms and cardiac myxoma. N Engl J Med 1970;282:35-6. [PubMed]
- Yaguchi H, Murakami Y, Sengoku R, et al. A case of cardiac myxoma presenting with multiple cerebellar hemorrhages and elevation of interleukin-6 in the cerebrospinal fluid. Rinshō shinkeigaku 2004;44:677-81. [PubMed]
- Stöllberger C, Finsterer J. Patients with cardiac myxoma require surveillance for myxoma-related cerebral aneurysms. Eur J Neurol 2008;15:e110-1. [PubMed]
- Orlandi A, Ciucci A, Ferlosio A, et al. Increased expression and activity of matrix metalloproteinases characterize embolic cardiac myxomas. Am J Pathol 2005;166:1619-28. [PubMed]
- Friedman DP, Rapoport RJ. Giant fusiform oncotic aneurysm: Mr and angiographic findings. AJR Am J Roentgenol 1996;167:538-9. [PubMed]
- Hwang BJ, Connelly MM, Lev MH. Distinctive MR imaging appearance of hemorrhagic cerebral aneurysms associated with atrial myxoma. AJR Am J Roentgenol 2001;177:925-7. [PubMed]
- Nucifora PG, Dillon WP. Mr diagnosis of myxomatous aneurysms: report of two cases. AJNR Am J Neuroradiol 2001;22:1349-52. [PubMed]
- Sonwalkar HA, Gupta AK, Ravi Varma D, et al. Cerebral Myxomatous Angiopathy. Imaging features. Rivista di neuroradiologia 2004;17:558-60.
- Nakata H, Yamada K, Hayakawa T, et al. A case of multiple cerebral aneurysms caused by cardiac myxoma. No Shinkei Geka 1985;13:1365-9. [PubMed]
- George KJ, Rennie A, Saxena A. Multiple cerebral aneurysms secondary to cardiac myxoma. Br J Neurosurg 2012;26:409-11. [PubMed]
- Walker MT, Kilani RK, Toye LR, et al. Central and peripheral fusiform aneurysms six years after left atrial myxoma resection. J Neurol Neurosurg Psychiatry 2003;74:281-2. [PubMed]
- Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610-7. [PubMed]
- Chiang KH, Cheng HM, Chang BS, et al. Multiple cerebral aneurysms as manifestations of cardiac myxoma: Brain imaging, digital subtraction angiography, and echocardiography. Tzu Chi Medical Journal 2011;23:63-5.
- Roeltgen DP, Weimer GR, Patterson LF. Delayed neurologic complications of left atrial myxoma. Neurology 1981;31:8-13. [PubMed]
- Bernet F, Stulz PM, Carrel TP. Long-term remission after resection, chemotherapy, and irradiation of a metastatic myxoma. Ann Thorac Surg 1998;66:1791-2. [PubMed]
- Yilmaz MB, Akin Y, Güray U, et al. Late recurrence of left atrial myxoma with multiple intracranial aneurysms. Int J Cardiol 2003;87:303-5. [PubMed]


