Deep sternal wound infection after cardiac surgery: a comparison of three different wound infection types and an analysis of antibiotic resistance
Introduction
Post sternotomy deep sternal wound infection (DSWI, also known as mediastinitis) is a severe complication of cardiac surgery which involves the deep-seated retrosternal tissues and mediastinal fascia. In recent series of reports, the incidence of DSWI was reported to be in the range 0.6–5% (1-5). Despite adequate surgical and medical treatment, DSWI causes longer hospital stays and increased cost, with associated mortality ranging from 10% to 47% (1-5).
The pathogenesis of DSWI involves a complex multifactorial process that includes a number of risk factors and pathogenic microorganisms. Several risk factors, dependent on both the patient and procedure, have been identified for DSWI. Preoperative or patient factors include age, gender, obesity, smoking, diabetes and respiratory insufficiency. Intraoperative factors such as emergent surgery, surgical type, and length of surgery have also exhibited an association with DSWI. Furthermore, post-operative factors such as prolonged ventilation, postoperative bleeding, re-operation, and requirement for transfusion have also been considered important to DSWI development (1,6-10).
The most common pathogens isolated in patients with DSWI are staphylococci and gram-negative bacteria (GNB) (6-8). However, various pathogenic microorganisms may lead to DSWI via distinct mechanisms. For example, DSWI associated with obesity, chronic obstructive pulmonary disease (COPD), and sternal dehiscence is often due to coagulase negative staphylococci (CNS) infection. Alternatively, DSWI following perioperative contamination of the mediastinal space is often caused by S. aureus, while DSWI associated with bacterial spread from other sites is often caused by GNB (6).
Although numerous studies have focused on analysis of risk factors and prevention strategies for the development of DSWI, only a few studies have focused on associations between time of onset and clinical features and outcomes. Specifically, DSWI may display variable features in terms of pathophysiology, clinical presentation, and microbial etiologies that may correlate with time to onset (7). In a previous study, Pairolero and Arnold (11) classified DSWI into three types that are now widely used for DSWI classification and are based on the postoperative time of establishment of the infection. The present study aims to compare clinical features, etiological distribution and bacterial drug resistance among the three distinct types of DSWI to guide the future development of preventive and therapeutic strategies for DSWI.
Methods
Study population
From 2011 to 2015, 170 adult patients with secondary DSWI occurring after cardiac surgery were admitted to our department, where they underwent pectoralis major muscle flap transposition surgeries. The diagnostic time of DSWI was defined as the interval between the initial sternotomy and either the time of DSWI diagnosis by the attending physician or the time of onset of the earliest recorded clinical manifestation (12). This study protocol followed ethical guidelines and was approved by the Bioethics Committee of our hospital (approval number: HK2017-01-10).
Definitions of DSWI
As defined by the Centers for Disease Control and Prevention (13), DSWI diagnosis requires at least one of the following criteria: (I) an organism is isolated from culture of mediastinal tissue or fluid; (II) evidence of mediastinitis seen during surgery; (III) one of the following conditions: chest pain, sternal instability, or fever (>38 °C) in combination with either purulent discharge from the mediastinum or isolation of an organism from culture of blood or mediastinal drainage.
Classification of DSWI
Patients who developed DSWI were classified according to the criteria proposed by Pairolero and Arnold (11). This classification system divides DSWI into three types based on duration and clinical findings. Type I infections occur in patients with wound separations with or without sternal instability that develops within a few days of sternotomy in the absence of mediastinal suppuration. Type II infections, the majority of cases, occur within the first few weeks after sternotomy and usually involve cellulitis, purulent wound drainage, and obvious communication with the sternum and mediastinum; costochondritis is rare, but osteomyelitis is common. Type III infections typically involve a chronically draining sinus tract into the sternum or costochondral arches within several months after the initial cardiac surgery.
Surgical management
We assembled a multidisciplinary team in our department that included cardiac surgeons, anesthesiologists, cardiologists, critical medical specialists and nurses. Using a team approach, we have implemented or refined pre-, peri- and post-operative processes to prevent and control infection. The implementations included: optimized perioperative analgesia, maintenance of hemodynamic stability, nutritional support, surveillance and control of perioperative glycemia (<200 mg/dL), perioperative antibiotic choice, operating room improvements, improvement of the anesthesia program, shortening of the operation time, minimization of blood product usage, post-operative wound care, and patient education regarding prevention of incisional infection.
Surgical treatment consisted of an exploratory thoracotomy that incorporated use of several swabs to collect samples for bacteriological analysis. Next, any infected tissues (the incision and surrounding soft tissues, granulation tissue, dead bone tissue) and steel wire or sutures were removed thoroughly. After surgical debridement, the muscle flap (the pectoralis major muscle and/or the rectus abdominus muscle flap) was used to close the infected area. Finally, drainage was aspirated using a negative pressure bottle connected to three to five Redon catheters inserted into infected areas, including the mediastinal cavity, muscle flap and dissected subcutaneous chest wall area (14). The effluents collected in separate bottles were bacteriologically cultured three times per week. The catheters were progressively removed after two consecutive negative culture results if the daily effluent volume was less than 5 mL (15). In addition, patients are usually admitted to the intensive care unit (ICU) and remained intubated and ventilated for the first 24 hours.
Microbiological methods and antibiotic therapy
For each patient, direct examination of samples and cultures with Gram-staining was performed. If dehiscence was observed, cultures of purulent exudates were obtained from surgical wounds or from the mediastinum during surgical intervention. Needle aspiration of the anterior mediastinal space was employed when a diagnosis of sternal infection was highly suspected but not established by other means. Specimens taken during surgery were transported immediately to the bacteriology laboratory in a closed syringe or blood culture bottle. Wound specimens were incubated under aerobic and anaerobic conditions, and isolates were identified using standard microbiological techniques (12,15).
The initial antibiotic therapy regimen was determined based on a direct examination of Gram-staining. Vancomycin was administered when Gram-positive cocci (GPC) were identified, while β-lactams were administered when GNB were identified. When Gram-staining was not conclusive, β-lactams and vancomycin were administered. The choice of β-lactams depended on host factors, including the risk for infection with antibiotic-resistant pathogens and observed local susceptibility patterns. This therapy was subsequently modified as needed according to the antibiograms of suspected causative microorganisms. Antibiotic therapy was maintained for six weeks after the last surgical debridement (15).
Data collection
For each patient, hospital records and microbiology laboratory records were reviewed. The following variables were recorded: (I) the patient risk factors included age, gender, smoking, COPD, hypertension, diabetes, hyperlipidemia, preoperative renal failure, immunosuppressive treatment, myocardial infarction, and cardiac functional classification according to the New York Heart Association (NYHA); (II) type of cardiac surgery performed included coronary artery bypass grafting (CABG), valve replacement, combined valve/CABG, aortic surgical procedures and other; (III) outcome data (from our department) included length of ICU stay, total days of hospital stay and hospitalization costs; (IV) microbiological results included characteristics of pathogens isolated from wounds and drug sensitivity tests.
Statistical analysis
Statistical analyses were carried out using SPSS 21.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative variables were described using the mean (± SD) for normally distributed data, the median [interquartile range (IQR)] for non-normally distributed data and frequency and percentage for qualitative variables. Quantitative variables were compared using the Kruskal-Wallis test or Mann-Whitney U test. Qualitative variables were compared using the Chi-square or Fisher’s exact test. Values of P<0.05 were considered significant.
Results
Classification of DSWI
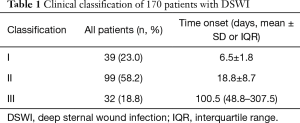
A total of 170 patients sustained a DSWI. The median time to onset (IQR) of DSWI was 15 days (range 10–30 days) over the entire series, with 39 patients developing type I DSWI (mean time to onset 6.5±1.8 days), 99 patients developing type II DSWI (mean time to onset 18.8±8.7 days), and 32 patients developing type III DSWI (median time to onset 100.5 days; Table 1).
Full table
Epidemiologic characteristics
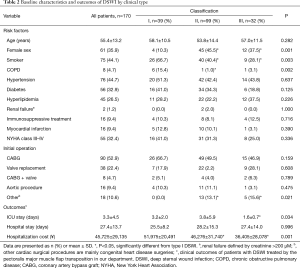
Table 2 shows the epidemiologic characteristics of the three types of DSWI patients. Among these 170 DSWI patients, 61 (35.9%) were females and the mean age for all patients was 55.4±13.2 years. There was a significantly greater proportion of women in type II vs. type I DWSI patients, as indicated by patient numbers of 45 (45.5%) vs. 4 (10.3%) (P=0.001), respectively, and in type III vs. type I patients, as indicated by patient numbers of 12 (37.5%) vs. 4 (10.3%) (P=0.018), respectively; however, the proportion of women did not differ between types II and III groups (P=1.000). Meanwhile, the proportion of smokers was higher in type I vs. type II DSWI patients, as indicated by patient numbers of 26 (66.7%) vs. 40 (40.4%) (P=0.015) and in type I vs. type III patients, as indicated by patient numbers of 26 (66.7%) vs. 9 (28.1%) (P=0.003), respectively; however, the proportion of smokers did not differ between types II and III DSWI patients (P=0.636). Compared with type II DSWI, the proportion of patients with COPD in type I DSWI was significant (P=0.006), but there was no significant difference in COPD rate between types I and III DSWI patients (P=0.555).
Full table
Details of the initial surgical procedure are also reported in Table 2. There was a significantly greater proportion of patients who underwent other cardiac surgical procedures in the types II and III DSWI groups as compared with type I DSWI patients: 13 patients (13.1%) vs. 0 patients (0.0%) (P=0.040) and 5 patients (15.6%) vs. 0 patients (0.0%) (P=0.036). However, the proportion of other cardiac surgical procedures did not differ between types II and III DSWI patients (P=0.951).
Notably, there was a significant difference in length of ICU stay and hospitalization cost in patients with type I DSWI compared to those with type III DSWI: 3.2±2.0 vs. 1.6±0.7 days (P=0.041) and ¥51,975±20,491 vs. ¥36,405±28,078 (P=0.001), respectively. Moreover, although statistically insignificant, a trend toward longer length of ICU stay and greater hospitalization cost was observed for patients with type II DSWI compared to type III DSWI (P>0.05).
Clinical features
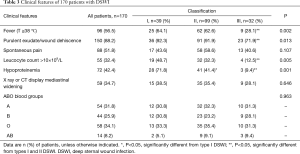
The most common initial symptoms in DSWI patients were fever and wound dehiscence accompanied by purulent secretions (Table 3). Compared with type III DSWI, the proportions of individuals with fever, purulent exudate with wound dehiscence, and elevated leucocyte count were higher in types I and II DSWI (P<0.05), but no significant difference was observed between types I and II DSWI (P>0.05). Meanwhile, the proportion of individuals with hypoproteinemia was higher in type I DSWI than in types II and III DSWI (Table 3; P<0.05). Notably, with regard to type III DSWI, which often develops after a delay of greater than one month, some patients only exhibited local symptoms such as spontaneous pain, thus illustrating that symptoms may be difficult to detect in type III DSWI.
Full table
Finally, Table 3 also shows the distribution of blood groups observed in the study population. Although there was no significant difference in distribution of blood groups in the three types of DSWI, there was an apparent trend of association of 170 patients with blood groups A and O with DSWI.
Microbiological characteristics
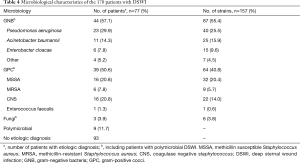
Microbiological diagnosis was available for isolates collected from 77 of 170 patients (45.3%) and 157 pathogens were identified in those patients (Table 4). In the GNB group, 87 pathogens were isolated. The most common GNB associated with mediastinitis were P. aeruginosa (n=40, 25.5%), followed by A. baumannii (n=25, 15.9%), Enterobacter cloacae (n=15, 9.6%), and other GNB pathogens (n=7, 4.5%) (Table 4).
Full table
Among 77 patients with definite pathogens, 68 were monomicrobial and 9 were polymicrobial. Polymicrobial mediastinitis was observed involving two microorganisms in each of five patients: MSSA and Escherichia coli (n=1), MRSA and Proteus mirabilis (n=1), MRSA and P. aeruginosa (n=1), CNS and P. aeruginosa (n=2), CNS and A. baumannii (n=2), CNS and E. cloacae (n=1), and P. aeruginosa and Candida albicans (n=1).
Although no statistical differences were found, GNB were documented more frequently in patients with types I and II DSWI. Meanwhile, GPC were more common in patients with types II and III DSWI, while polymicrobial infections more frequently associated with type II DSWI (Table 5).

Full table
Antibiotic resistance of bacterial isolates
The susceptibility patterns of three common GNB isolated from wound infections and tested against 13 selected antimicrobial agents (Table 6). The antibiotic susceptibility test results showed that P. aeruginosa isolates exhibited 100% resistance to cefazolin and cefuroxime, but were least resistant to ceftazidime (15.0%), imipenem and meropenem (17.5%). Meanwhile, the resistance rate of A. baumannii isolates to commonly used antibiotics was greater than 70%.
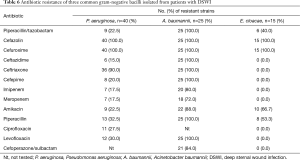
Full table
Staphylococcal isolates were tested against 11 antibiotics (Table 7). The drug resistance patterns of staphylococci isolated from wound infections were found to be highly variable, with resistance rates to penicillin-G of 100% and to clindamycin of over 70%. No staphylococcus isolates were resistant to vancomycin, linezolid and tigecycline.
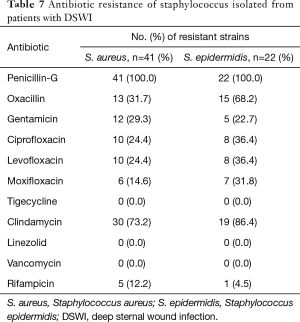
Full table
Discussion
DSWI following cardiac surgery is a serious and dreaded complication, with DSWI patients classified into three DSWI types according to criteria proposed by Pairolero and Arnold (11). Based on Pairolero classification, we compared the clinical features and etiological characteristics of the three DSWI types, with a view to further study the clinical significance of DSWI typing in our institution.
Our study showed that DSWI occurred most frequently within a month after cardiac surgery, with patients exhibiting type II DSWI comprising the largest group observed (58.2%, 99/170). We speculated that the duration of the DSWI incubation period in these patients may reflect processes acting after major surgery. Specifically, such timing reflects the fact that surgery, especially with cardiopulmonary bypass, can lead to systemic inflammatory response syndrome and its anti-inflammatory response (16) and many such changes can persist for long periods of time after cardiac surgery. We speculated that DSWI could have longer incubation times in those patients whose immune defenses were least affected. In addition, prophylactic use of preoperative antibiotics can assist the host's natural defense function and thereby prolong the incubation period, causing DSWI to occur as long as months after cardiac surgery. Conversely, DSWI may occur earlier and more frequently in those patients who have the most profound immune-paralysis.
Other researchers have also focused on the influence of patient-dependent factors on development of DSWI. In one study, Mekontso Dessap et al. (7) showed that patients with early-onset DSWI often needed postoperative surveillance in an ICU and were more likely to develop life-threatening complications. Our results also confirmed that type I DSWI patients, with their greater likelihood of smoking and COPD than for other DSWI groups, were associated with longer ICU stays and incurred higher hospitalization cost. Furthermore, we found that for types II and III patients, the proportion of female patients was higher than observed for type I patients. Our results therefore aligned with those of Charbonneau et al. (15), that demonstrated female gender as an independent risk factor of GNB mediastinitis. They hypothesized that increased risk in women was due to the fact that women have higher prevalence rates of GNB urinary tract infection or colonization, but they presented no evidence to support their hypothesis.
In this study, we found that both types I and II DSWI patients showed typical clinical symptoms such as fever, purulent exudate with wound dehiscence, spontaneous pain, and high leucocyte count, as well as a more frequent association with hypoproteinemia (P=0.001). However, some patients with delayed type III DSWI developing after more than one month only exhibited local symptoms such as spontaneous pain. Because type III DSWI onset was significantly delayed and clinical manifestations were not typical, the diagnosis and treatment in such cases are also delayed. Therefore, for a severely ill patient with previous cardiac surgery and multiple risk factors for poor wound healing, the clinician must maintain a high level of suspicion for DSWI despite delayed presentation, so as not to delay treatment (17). Furthermore, we also explored the distribution frequency of ABO blood group in the three types of DSWI. Although there was no significant difference in distribution of blood groups among the three types of DSWI, there was an apparent trend of association of patients with blood groups A and O with DSWI, that requires further study.
Regarding the sensitivity of detection in this study, the detection rate of pathogens in patients with DSWI was only 45.3%, lower than that of other reports (1,7,8,15). There are several reasons why this might be the case, including the following: (I) blood culture results were not included in our study; (II) 170 patients who suffered DSWI had undergone cardiac surgery in many hospitals throughout the country, with DSWI occurring after cardiac surgery. Quite a few of the 170 patients had been treated with broad-spectrum antibiotics during previous hospitalizations before entering our hospital to receive the pectoralis major muscle flap transposition. However, we were unable to obtain detailed etiological data of these patients from the other hospitals; (III) half of the samples for each patient were placed into closed syringes, which may have reduced the culture positivity rate; (IV) the delay between sample collection during surgery and the placing of those samples into culture media in the laboratory may have reduced the counts of culturable organisms within the collected samples (18); (V) atypical organisms are involved in DSWI that are difficult to isolate in routine cultures.
The microbiological characteristics of DSWI in this study are not like those previously reported (1,6,8,15). Here, 157 pathogens were identified in the 77 patients, of which 55.4% were GNB and of these the most common were P. aeruginosa (25.5%). In addition, the results of this study suggest that GNB were documented more frequently in patients with types I and II DSWI, while GPC were more common in patients with types II and III DSWI. However, polymicrobial infections more frequently associated with type II DSWI, although no statistical differences were found. Because the distribution of pathogenic microorganisms tends to vary in different countries, such differences result from the actions of several factors, such as local bacterial ecology, institutional antibiotic policy, or both. Therefore, these variations could partially explain the discrepancies between pathogen profiles reported in numerous previous studies (15).
Because antibiotic resistance is becoming more prevalent and is of growing concern, we must also discuss it here. In this study, P. aeruginosa isolates showed 100% resistance to cefazolin and cefuroxime, and showed least resistance to ceftazidime (15.0%), cefepime (20.0%), imipenem and meropenem (17.5%). The emergence of antibiotic-resistant P. aeruginosa is increasing globally due to antibiotic overuse. In addition, the percentage of the multidrug-resistant (MDR) or extensively drug-resistant (XDR) P. aeruginosa isolates has increased over the years and greatly limits therapeutic options. A major reason for this tendency is that P. aeruginosa has high intrinsic resistance to a wide range of antimicrobial agents and can also develop resistance during therapy (19).
Also of great concern, A. baumannii isolates have become resistant to almost all currently available antibiotics, including (carbapenems). Subsequently, several clinical outbreaks of carbapenem-resistant A. baumannii have been described (20-22) and therapeutic options for treatment of MDR A. baumannii are becoming increasingly limited.
Nosocomial infections are an additional mechanism of spread of multi-resistant pathogens. Specifically, members of the E. cloacae complex are important pathogens responsible for nosocomial infections. Although isolates of the E. cloacae were fully susceptible to almost all currently available antimicrobial agents in our study, E. cloacae could be induced to produce active and constitutive β-lactamase, resulting in nosocomial infection outbreaks by this organism. Moreover, the E. cloacae complex has also acquired resistance to another widely used class of antibiotics, the fluoroquinolones (23).
Finally, the antimicrobial resistance of MRSA has become of increasing clinical concern during the past few decades. However, the resistance rates of MRSA vary considerably among numerous reports. For example, Seidl et al. (24) found that MRSA isolates showed resistance to erythromycin (48%), ciprofloxacin (43%), clindamycin (31%), tetracycline (22%), and gentamicin (16%). In this study, all of our isolates were susceptible to vancomycin, with about 90% susceptible to rifampicin. Notably, in another study methicillin resistance did not significantly influence ICU mortality among patients with post-sternotomy mediastinitis who benefited from optimal treatments (25). S. epidermidis is the most common agents among CNS isolates; moreover, approximately 68% of the S. epidermidis isolates were methicillin resistant. Methicillin-resistant CNS isolates were determined to be more resistant to antibiotics than methicillin-susceptible CNS isolates. None of the isolates were resistant to vancomycin and teicoplanin (26). Sommerstein et al. (27) found that perioperative antimicrobial therapy, early re-intervention for noninfectious causes and time between surgery and diagnosis of infection over 21 days are each associated with methicillin resistance among DSWI.
To our knowledge, this is the first study in China to compare the epidemiology, clinical features, and microbiology of three types of DSWI. However, we also are aware of several limitations of our study. First, the results of this study are derived from retrospective data analysis of patients admitted to a single medical center. Second, we did not include information related to the initial surgeries, including CPB time, cross-clamp time, length of first operation, perioperative antibiotic prophylaxis, and technique of sternal closure. Third, this study might have yielded different results if we had included results of blood cultures and microbial cultures of remote-site infections (e.g., pulmonary and urinary infections), since postoperative remote-site infections may also lead to mediastinitis, especially if GNBs are involved (12).
In conclusion, the results obtained in the present study support the classification of DSWI into three types according to both the Pairolero classification standard and also from epidemiological and clinical characteristics distinguishing among these types. Identification of the most common pathogens as GNB and staphylococcus should inform treatment. However, evidence of increasing antibiotic resistance in pathogens infecting DSWI patients is a great concern.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol followed ethical guidelines and was approved by the Bioethics Committee of our hospital (approval number: HK2017-01-10).
References
- Floros P, Sawhney R, Vrtik M, et al. Risk factors and management approach for deep sternal wound infection after cardiac surgery at a tertiary medical centre. Heart Lung Circ 2011;20:712-7. [Crossref] [PubMed]
- Losanoff JE, Richman BW, Jones JW. Disruption and infection of median sternotomy: a comprehensive review. Eur J Cardiothorac Surg 2002;21:831-9. [Crossref] [PubMed]
- Gummert JF, Barten MJ, Hans C, et al. Mediastinitis and cardiac surgery—an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg 2002;50:87-91. [Crossref] [PubMed]
- Ridderstolpe L, Gill H, Granfeldt H, et al. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168-75. [Crossref] [PubMed]
- Baillot R, Cloutier D, Montalin L, et al. Impact of deep sternal wound infection management with vacuum-assisted closure therapy followed by sternal osteosynthesis: a 15-year review of 23,499 sternotomies. Eur J Cardiothorac Surg 2010;37:880-7. [Crossref] [PubMed]
- Gårdlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery - microbiology and pathogenesis. Eur J Cardiothorac Surg 2002;21:825-30. [Crossref] [PubMed]
- Mekontso Dessap A, Vivier E, Girou E, et al. Effect of time to onset on clinical features and prognosis of post-sternotomy mediastinitis. Clin Microbiol Infect 2011;17:292-9. [Crossref] [PubMed]
- Chen LF, Arduino JM, Sheng S, et al. Epidemiology and outcome of major postoperative infections following cardiac surgery: risk factors and impact of pathogen type. Am J Infect Control 2012;40:963-8. [Crossref] [PubMed]
- Singh K, Anderson E, Harper JG. Overview and management of sternal wound infection. Semin Plast Surg 2011;25:25-33. [Crossref] [PubMed]
- Lepelletier D, Bourigault C, Roussel JC, et al. Epidemiology and prevention of surgical site infections after cardiac surgery. Med Mal Infect 2013;43:403-9. [Crossref] [PubMed]
- Pairolero PC, Arnold PG. Management of infected median sternotomy wounds. Ann Thorac Surg 1986;42:1-2. [Crossref] [PubMed]
- Rodríguez-Hernández MJ, de Alarcón A, Cisneros JM, et al. Suppurative mediastinitis after open-heart surgery: a comparison between cases caused by Gram-negative rods and by Gram-positive cocci. Clin Microbiol Infect 1997;3:523-30. [Crossref] [PubMed]
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250-78. [Crossref] [PubMed]
- Liu D, Wang W, Cai A, et al. Analysis of surgical treatment with pectoralis major muscle flap for deep sternal infection after cardiac surgery: a case series of 189 patients. Zhonghua Wai Ke Za Zhi 2015;53:193-6. [PubMed]
- Charbonneau H, Maillet JM, Faron M, et al. Mediastinitis due to Gram-negative bacteria is associated with increased mortality. Clin Microbiol Infect 2014;20:O197-202. [Crossref] [PubMed]
- Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect 2006;8:1382-9. [Crossref] [PubMed]
- Joseph L, Jeanmonod RK. Delayed presentation of deep sternal wound infection. West J Emerg Med 2014;15:134-6. [Crossref] [PubMed]
- Blackmur JP, Tang EY, Dave J, et al. Use of broth cultures peri-operatively to optimise the microbiological diagnosis of musculoskeletal implant infections. Bone Joint J 2014;96-B:1566-70. [Crossref] [PubMed]
- Hancock RE, Speert DP. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 2000;3:247-55. [Crossref] [PubMed]
- Hamzeh AR, Al Najjar M, Mahfoud M. Prevalence of antibiotic resistance among Acinetobacter baumannii isolates from Aleppo, Syria. Am J Infect Control 2012;40:776-7. [Crossref] [PubMed]
- Meric M, Kasap M, Gacar G, et al. Emergence and spread of carbapenem-resistant Acinetobacter baumannii in a tertiary care hospital in Turkey. FEMS Microbiol Lett 2008;282:214-8. [Crossref] [PubMed]
- Valenzuela JK, Thomas L, Partridge SR, et al. Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii. J Clin Microbiol 2007;45:453-60. [Crossref] [PubMed]
- Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol 2012;7:887-902. [Crossref] [PubMed]
- Seidl K, Leimer N, Palheiros Marques M, et al. Clonality and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus at the University Hospital Zurich, Switzerland between 2012 and 2014. Ann Clin Microbiol Antimicrob 2015;14:14. [Crossref] [PubMed]
- Combes A, Trouillet JL, Joly-Guillou ML, et al. The impact of methicillin resistance on the outcome of poststernotomy mediastinitis due to Staphylococcus aureus. Clin Infect Dis 2004;38:822-9. [Crossref] [PubMed]
- Koksal F, Yasar H, Samasti M. Antibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol Res 2009;164:404-10. [Crossref] [PubMed]
- Sommerstein R, Kohler P, Wilhelm MJ, et al. Factors associated with methicillin-resistant coagulase-negative staphylococci as causing organisms in deep sternal wound infections after cardiac surgery. New Microbes New Infect 2015;6:15-21. [Crossref] [PubMed]


