Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients
Introduction
Immune checkpoint inhibitors (ICIs) such as antagonistic monoclonal antibodies against programmed death-1 (PD-1) nivolumab and pembrolizumab; the programmed death ligand-1 (PD-L1) atezolizumab, durvalumab and avelumab; and against cytotoxic T-lymphocyte antigen 4 (CTLA-4) ipilimumab and tremelimumab, have changed treatment paradigm of advanced non-small cell lung cancer (NSCLC) and other thoracic malignancies. In first-line setting, both FDA and EMA, on October 2016 and on January 2017, approved pembrolizumab in NSCLC tumours with strong PD-L1 expression (PD-L1 ≥50%) based on significant improvement in progression free survival (PFS) and overall survival (OS) and fewer adverse events (AE’s) compared with platinum-based chemotherapy (1). As contrary, upfront nivolumab did not improve PFS or OS compared with platinum-based chemotherapy among patients with NSCLC with a PD-L1 expression level of 5% or more (2). In first-line treatment, Atezolizumab activity has also been explored in PD-L1 positive advanced NSCLC patients (BIRCH trial) with encouraging activity mainly related to those patients with highest PD-L1 expression (3). Finally, in a multicohort phase I trial, upfront avelumab as monotherapy has also reported durable clinical activity and a confirmatory phase III trial is currently ongoing (NCT02576574) (4). Combinations of ICIs as first-line treatment have been also evaluated (5). However, the three arms phase III randomized MYSTIC trial with durvalumab and tremelimumab in first-line did not achieved its primary endpoint to demonstrate significant improvement in PFS compared to chemotherapy in patients with tumours expressing PD-L1 of 25% or higher. Of note, combinations of anti-CTLA-4 and anti-PD-1 in NSCLC patients have reported increased incidence of grade ≥3 AE’s (5), so, the ratio between efficacy/toxicity and dose of the ICIs need to be considered when testing combinations (6). Finally, combinations of chemotherapy and ICIs have also been evaluated. In a recent phase II trial, upfront combination of pembrolizumab, carboplatin and pemetrexed significantly improved RR and PFS compared with standard of care chemotherapy in advanced non-squamous NSCLC patients irrespective of PD-L1 expression (7). Based on these results, the combination received FDA accelerated approval on 10th May 2017 contingent upon verification of the clinical benefit in other confirmatory phase III trials: the KEYNOTE-189 (NCT02578680) in non-squamous, and the KEYNOTE-407 (NCT02775435) in squamous NSCLC patients. It is worth mentioning that in squamous NSCLC patients, the addition of ipilimumab to first-line chemotherapy did not prolong OS compared with chemotherapy alone (8).
In second-line treatment, four randomized phase III clinical trials have demonstrated a statistically significant improvement in OS with nivolumab (9,10), pembrolizumab (inclusion restricted to patients with at least 1% PD-L1 expression on tumor cells) (11), and atezolizumab (12) compared to docetaxel. The magnitude of benefit with pembrolizumab (11) and atezolizumab (12) was improved among patients whose tumours overexpress PD-L1. Both, the FDA and EMA have approved all three drugs in second-line setting. In the absence of head-to-head comparisons and similar biological activity as well as toxicity profile (13,14), it is not possible to recommend one treatment over another. Avelumab has also demonstrated clinical activity and acceptable safety profile in a phase Ib trial with previously treated advanced NSCLC patients (15). In the phase III PACIFIC trial, maintenance treatment with durvalumab for 1 year after chemo-radiotherapy in locally advanced NSCLC significantly improved PFS, RR and median time to death or development of distant metastases compared with placebo (16). Based on these results, durvalumab granted breakthrough therapy designation by the FDA on 31st July 2017 to treat locally advanced unresectable NSCLC patients after chemo-radiotherapy. Finally, in the early stages, different ongoing clinical trials are also evaluating the role of ICIs in the adjuvant setting (i.e., NCT02486718, NCT02504372, NCT02273375) or as induction (i.e., NCT02998528, NCT03081689, NCT02259621, NCT02994576, NCT02927301, NCT02716038, NCT02818920, NCT03030131).
ICIs have been also tested in other thoracic malignancies reporting preliminary activity like in malignant pleural mesothelioma with pembrolizumab (17,18), nivolumab (19,20), avelumab (21), tremelimumab (22), or the combination of nivolumab and ipilimumab (20); in small cell lung cancer (SCLC) with pembrolizumab (23,24), atezolizumab (25), or nivolumab plus ipilimumab (26); and in thymic epithelial tumours with pembrolizumab (27,28) and avelumab (29). However, their role in the therapeutic sequence for these malignancies remains to be defined.
The use of all these agents in different malignancies has changed the toxicity landscape known by oncologists and new spectra of immune related (ir-)AE’s has been reported. In this review, we will summarize the incidence and management of side effects related to ICIs focusing in NSCLC patients.
Incidence of immune-related systemic adverse events in NSCLC patients and in specific clinical conditions
In a phase I study of 129 treated NSCLC-patients possible autoimmune-based events of any grade occurred in 41% including skin, gastrointestinal (GI) and pulmonary events. These ir-AE’s were mostly low grade and if high grade manageable by discontinuation, immunosuppression treatment (steroids) or hormone replacement (30). Recently, a systematic review including 5,744 NSCLC patients from 23 studies treated with anti-PD-1 or anti-PD-L1 reported a global incidence of AE’s of 64% (14% grade ≥3) with anti-PD-1 and 66% (21% grade ≥3) with anti-PD-L1 agents, respectively (13). Fatigue was the most common toxicity reported. The incidence of ir-AE’s was of 16% (3% grade ≥3) in patients treated with an anti-PD-1 and 11% (6% grade ≥3) in patients with an anti-PD-L1, with a light increase risk of pneumonitis with anti-PD-1 inhibitors (13). The incidence of ir-AE’s in NSCLC patients treated with a combination of anti-PD-1 plus anti-CTLA-4 (5) or anti-PD-1 plus chemotherapy (7) is higher; 30% (5% grade ≥3) and 19% (4% grade ≥3), respectively (Table 1).
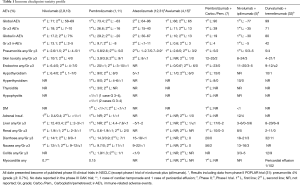
Full table
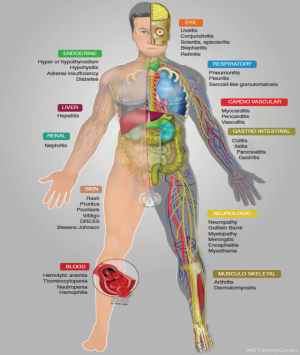
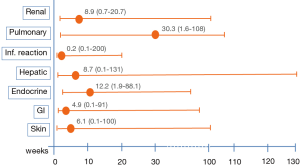
In general onset of ir-AE’s occur quite early, mostly within weeks to 3 months after initiation of ICIs, and nearly all organs can be affected (Figure 1). In some cases, the onset of ir-AE’s may be delayed up to 1 year after treatment initiation, specially pulmonary and hepatic toxicity (Figure 2) (33). Systemic ir-AE’s have been reported in 2.3% of patients, mostly moderate (grade 2, 63%) (34).
Elderly patients (≥65 years) represent most patients diagnosed with cancer and deaths by age group, and an increase is expected over the next decades (35). It is unclear whether elderly might exert primary resistance or higher risk of toxicity with immunotherapy, a phenomenon that might be linked to the continuous and progressive remodeling of immune functions with ageing, known as immunosenescence (36), and age-associated decrease of immune response (37). Indeed, in solid tumors, age beyond 65 is a risk factor for hyperprogressive disease on ICIs (38). However this observation has not been proven by others among NSCLC patients (39). Efficacy of ICI in elderly population is controversial. A meta-analysis reported similar OS benefit with ICIs in both, younger and older patients with a cut-off age of 65–70 years, with no survival benefit in patients beyond 75 years with anti-PD-1 (40). In a recent meta-analysis performed in NSCLC patients, the relative treatment benefit with ICIs were similar according to age <65 years (HR, 0.71) vs. ≥65 years (HR, 0.69); interaction (P=0.74) (41). Similarly, using 65 years as the age cut-off, the pooled HR with anti-PD(L)1 in NSCLC phase III clinical trials showed a 34% death risk reduction in favor of ICIs compared to chemotherapy (36). Elderly patients (≥70 years) with squamous NSCLC treated with nivolumab from the CheckMate 171 reported 46% of treatment related-AE’s and 5% of discontinuations due to AE’s (42). Toxicities were similar to those of the overall population included in the study.
In a FDA subset analysis, toxicity (grade 3–5) with anti-PD(L)1 seems similar between younger and older patients with a cut-off point of 65 years. However, patients beyond 70 years presented more grade 3–5 AE’s than patients <65 years (71.7% vs. 58.4%), along with higher AE’s leading discontinuation (19.8% vs. 14.4%) and immunosuppressive needs (51.9% vs. 41.5%) (43). The ir-AE’s may be more challenging in elderly population due to reduced functional reserve and pre-existence of comorbidities. Nevertheless, no dose adjustment for ICIs is recommended for elderly patients with mild or moderate renal (i.e., ≥30 mL/min creatinine clearance) or hepatic impairment (i.e., total bilirubin ≥ upper limit normal to 1.5 N). Also, cytochrome P450 enzymes do not metabolize the currently approved ICIs, so any enzymatic competition is not expected for elderly patients treated with polypharmacy (44).
Most clinical trials exclude patients with baseline autoimmune diseases, and rarely allow inclusion of those with well-controlled non-steroid dependent autoimmune diseases. In a retrospective cohort of 46 NSCLC patients treated with ICIs and autoimmune disease, exacerbation of any underlying autoimmune disease occurred in 17% of patients. The overall ir-AE’s in this population was 26% (11% grade 3–4), similar to that reported in patients without autoimmune disease at baseline (45). These results may suggest the safety and efficacy of PD-(L)1 inhibitors as therapeutic strategies in NSCLC patients with asymptomatic and controlled autoimmune diseases.
No clear data exist to guide decisions with regards retreatment with ICIs after the onset of an ir-AE’s. This is of relevance, as approximately 3% to 12% of patients will discontinue treatment with anti-PD-1 due to ir-AE’s (46). In a subset of retreated NSCLC patients after ir-AE’s, half did not experience any recurrence, 24% and 26% developed the same or a new toxicity and there was 5% related mortality. Moreover, there were no differences in OS between re-treated and no-re-challenged patients suggesting a correlation between ir-AE’s development and patients’ outcome (47). In melanoma patients, the onset of ir-AE’s correlate with the efficacy of ICIs (48), and recently, among different cohorts of advanced NSCLC patients, development of ir-AE’s was also significantly associated with longer OS (49-51). However, more prospective research is warranted to validate this correlation.
Whether dose of ICIs could have an impact in the late ir-AE’s remains unknown. Recently, nivolumab (52) and pembrolizumab (53) have adopted flat schedule in place of the standard weight-adjusted dose in some clinical trials and it has been selected as the standard dose in the first-line setting with pembrolizumab (1). The FDA accepted application for flat dose of nivolumab (480 mg Q4W) on 24 July 2017. However, there are some challenges regarding flat dose strategy such as the economic impact for those patients overdose (54), and whether the incidence, grade or unexpected late systemic ir-AE’s will increase. Therefore, close monitoring and long follow-up of these patients in clinical trials is warranted.
Dysimmune toxicities: diagnosis, treatment and follow-up
Clinical management and diagnosis of ir-AE’s is new and expertise is still limited. These aspects emphasize the importance of close collaboration between oncologist and other specialists. Five principles of clinical management of ir-AE’s have been recently proposed (44,55) and should be applied in daily clinical practice (Figure 3): prevent, anticipate, detect, treat, and monitor.
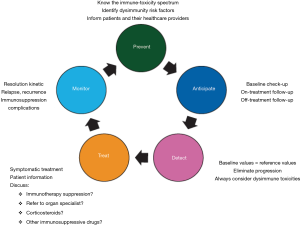
Mechanisms of toxicity remain to be defined and may well be heterogeneous between patients even when the same agent is used. Patterns of ir-AE’s differ according to the immune checkpoint subtype, being colitis, hypophysitis and rash more frequent with CTLA-4 monoclonal antibodies, whereas pneumonitis, hypothyroidism, arthralgia and vitiligo appear to be more common with PD-1 monoclonal antibodies (46). Recently different guidelines have been published for the management of toxicities from immunotherapy (55,56). Here, we aim to summarize the spectrum of dysimmune toxicities (ir-AE’s) focusing on NSCLC patient, as well as specific management recommendations, which have been summarized in Table 2. In case of no clinical improvement after 3 to 5 days of treatment initiation, upgrade treatment recommendations (55). Main treatment for ir-AE’s is steroids. Tapering of steroids should be performed very slow and careful, over 6 weeks or more, to avoid relapses of ir-AE’s during steroid tapering (56,57). Consider Pneumocystis prophylaxis in patients receiving long-lasting steroids therapy.

Full table
Pneumonitis
The incidence, timing, clinical features and outcomes of pulmonary ir-AEs are quite variable. Pneumonitis is an uncommon but clinically serious and potential life-threating adverse event. In a recent meta-analysis of 4,496 patients who were treated in 20 trials of PD-1 inhibitors for melanoma, NSCLC (5 studies), and renal cell carcinoma, the overall incidence of pneumonitis was 2.7% (0.8% grade ≥3) during monotherapy and 6.6% during combination. The incidence of pneumonitis all-grade was higher in NSCLC (4.1% vs. 1.6%, P=0.002), as well as grade ≥3 (1.8% vs. 0.2%, P<0.001) compared with melanoma. Pneumonitis was more frequent in combination therapy than with monotherapy (all-grade 6.6% vs. 1.6%, P<0.001; and grade ≥3 1.5% vs. 0.2%, P=0.001) (58). In a recent retrospective analysis incidence of pneumonitis was of 3.5% among 1,826 cancer patients treated with ICIs. It occurred mainly in former- or current-smokers, with grade 4 in 9.4% and up to 10% of fatal pneumonitis (59). The onset of pneumonitis occurred from 2.3–2.8 months after treatment initiation (57,59,60). Onset tended to occur earlier in NSCLC patients compared to melanoma (2.1 vs. 5.2 months, P=0.02) (59) or other malignancies (60). Nowadays, any clear association between PD-L1 expression and pulmonary toxicity remains elusive and identification of risk factors have not been reported (59).
In a report of long-term safety phase I trial in NSCLC treated with nivolumab, pneumonitis occurred in 7% (9 of 129 patients), with three pneumonitis related deaths (30). However, incidence reported from phase III clinical trials with PD-1/PD-L1 inhibitors in NSCLC ranges from 2.6% to 5.8%, with grade ≥3 from 1% to 2.6%. Frequency of fatal pneumonitis is low (0.3%) and discontinuation due to pneumonitis ranges from 1.1% to 2.6% (1,2). In a phase I trial, combination of nivolumab with platinum-based chemotherapy as first-line treatment of advanced NSCLC, pneumonitis was noted in 13% (7 of 56 patients) of patients, including 7% (4 of 56) with grade 3 to 4, and was the most common adverse event responsible for treatment discontinuation [3 (5%) of 56] (61). Also, another phase I trial combining of durvalumab and tremelimumab, reported 5% of drug-related pneumonitis, including four patients (4%) with grade 3 events (32), similar to that of the combination of nivolumab and ipilimumab in first-line treatment in advanced NSCLC patients (5) (Table 1). Also among SCLC patients, incidence of pneumonitis is higher with combination of ICIs compared with monotherapy (all grades: 4% vs. 2%, grade ≥3: 2% vs. 1%, respectively) (62). Combination of osimertinib and durvalumab in EGFR-mutant NSCLC patients, reported an incidence of 38%, which was much higher than would be expected with either drug alone, including five patients (15%) with grade 3–4 events (63).
In NSCLC patients, systematic review (13) and meta-analysis (64) have reported that pneumonitis would be more common with PD-1 inhibitors compared to PD-L1 inhibitors, and it was more common among treatment naïve patients (64). However, in a retrospective study of 915 patients (209 NSCLC) who were treated with PD-1/PD-L1 inhibitors, the incidence of pneumonitis was 5% in all tumors and 4.3% in NSCLC patients, greater with CTLA4-inhibitors combinations than monotherapy (10% vs. 3%, P<0.001); but no differences observed between patients who were treated with PD-1 or PD-L1 inhibitors (as monotherapy: 4% vs. 1% P=0.13; or in combination: 10% vs. 5%, P=0.7) (57). Pneumonitis grade ≥3 was reported in 27% of cases without differences in severity between monotherapy or combination, and 86% of patients improved or resolved with drug withholding and immunosuppression. Among patients who underwent re-challenge after resolution or improvement of pneumonitis grade 1–2, recurrent pneumonitis occurred in 25% of cases (57). In other series, the risk of recurrent pneumonitis increased up to 33% (47), so, retreatment is feasible but should be carefully individualized and patients carefully selected, especially in those early responders who develop an ir-AE’s, where retreatment does not clearly impact in outcome, and retreatment may increase the risk of death (47).
Risk of pneumonitis seems to be irrespective of smoking status, line of treatment; and chest radiation (57). Thoracic radiotherapy at least 6 months prior to ICIs treatment is an exclusion criteria in several clinical trials. However, in a recent retrospective review of 90 NSCLC patients, although pneumonitis was the most common ir-AEs (8.8%), history of thoracic radiation did not increase the risk of pneumonitis (11.5% vs. 8.3%, P=0.69), neither impact in OS outcomes (5.8 vs. 8.1, P=0.11) (49). In the PACIFIC trial (16), the risk of all grade pneumonitis with ICIs maintenance was 34% and grade ≥3 in 3.4% of patients (similar to those patients who received placebo). Lung cancer patients with idiopathic interstitial pneumonia have a higher risk of pneumonitis associated with anticancer therapy. However, in a pilot trial with nivolumab no concerns were observed in NSCLC patients with idiopathic interstitial pneumonia (65). However, more evidence is needed to corroborate this observation.
Pneumonitis is a toxicity of variable onset [in NSCLC patients can occur later than other ir-AE’s (33,57)], as well as in clinical, radiological and pathological features (59,60). These data, withstand the need of terminology standardization when describing radiological findings during of ICIs treatment. The commonly noted radiographic patterns of pneumonitis on ICIs are: cryptogenic organizing pneumonia like (COP-like), nonspecific interstitial pneumonia pattern, and acute interstitial pneumonia/acute respiratory distress syndrome pattern (66). COP-like appearance is more common among NSCLC patients (57).
Management
In NSCLC patients, disease progression or infection should always be ruled out. In general lung biopsy is recommended if there is radiological or clinical doubt about the etiology (disease, infection, toxicity) of pulmonary infiltrates. Alternatively, depending on the radiological pattern on CT scan, a bronchoscopy with bronchoalveolar lavage could be appropriate, especially in case of pneumonitis grade ≥2 (56). In case of no improvement to grade 2, high resolution CT is recommended (56,57). In grade 3–4 pneumonitis without improvement with steroids, additional immunosuppressive strategies should be implemented (infliximab 5 mg/kg, mycophenolate or cyclophosphamide).
Skin toxicity
Skin toxicity from ICIs, is an early ir-AE’s ranging from rash or pruritus to vitiligo (67). Despite that incidence may be underreported, skin AE’s are among the most common ir-AE’s with ICIs (68,69). Serious skin toxicity is rare (grade ≥3 is less than 4%) and does not require dose reduction or treatment discontinuation. Rash and pruritus are reported in 24% and 25–35%, respectively, of patients treated with ipilimumab (68), in 9–10% of patients treated with anti-PD-1 inhibitors (1,2,9-12), increasing to 15% and 25% with the anti-PD-1 and anti-CTLA-4 or chemotherapy combination (5,7,32). Recently, atezolizumab showed pruritus at 8% (<1% grade >3) (12). However, avelumab only induced one case (1%) reported as psoriasis in pretreated patients (4,15). There are no global differences between anti-PD-1 and anti-PD-L1 inhibitors (13). Among melanoma patients, the occurrence of vitiligo seems to be related with a better overall response rate (67), but this has not been reported among NSCLC patients. Other skin toxicity have been rarely reported: liquenoid eruptions (70), bullous pemphigoid (71) and Grover disease (72).
Management
Treatment management according Table 2. The first requirement in the skin toxicity management should be to rule out any other skin toxicity aetiology, such as an infection, drug interaction or another systemic disease (73). A complete physical examination needs to be performed, including mucosal areas for defining the body surface area (BSA) affected by the toxicity; and a blood test with liver and kidney function. Grade 1 and 2 rash are defined as macules/papules covering <10% or 10–30% BSA with or without symptoms (56). Treatment based on topical emollients, oral antihistamines and/or topical mild strength topical steroids is recommended with no immunotherapy interruption required. For grade 3 skin toxicity (>30% BSA), referral to the dermatologist and perform a skin biopsy should be strongly considered in this situation. Grade 4 skin rash is defined as: papulopustular rash associated with life-threatening infection, Steven-Johnson syndrome, toxic epidermal necrolysis and bullos dermatitis covering ≥30% of BSA, requiring intensive care unit; and systemic steroids are required according Table 2 and biopsy is mandatory (56).
Digestive toxicity
GI toxicity is well defined with anti-CTLA-4 inhibitors but not with anti-PD-1 or combination strategies.
Diarrhoea (defined as an increase in the frequency of stools) occurs in 20–32% of NSCLC patients treated with immunotherapy combination strategies at any grade, with a 11% grade 3–4 severe rate (5,7,32). Incidence decreases to half with anti-PD-1/PD-L1 inhibitors (less than 4% grade >3) (1,2,4,9-12,31). The median time from drug initiation to symptom onset is 3 months (74), although colitis may even occur several months after the last dose of ipilimumab (75).
Colitis is defined as a special immune-related GI AE’s, defined as abdominal pain or imaging and/or endoscopic evidence of colonic inflammation. Its frequency at any grade is around 1–1.9% with pembrolizumab (1,11) and atezolizumab (12), increasing the incidence with the anti-PD-1 and CTLA-4 combinations to 3–12%, with a 5% to 9% of grade >3 rate (5,7,32). While there is a positive association between ipilimumab-induced colitis and tumor regression or OS in melanoma patients (74,76), this phenomenon has not been described in NSCLC patients, neither ipilimumab nor anti-PD-1/PD-L1 inhibitors. Also, in melanoma patients, the sequence of anti-PD-1 followed by ipilimumab, or the reverse did not predict GI toxicity (77).
Nausea is the second most frequent GI symptom, although not always has been reported as ir-AE’s (78) described in about 9–22% of patients. There were no global differences according to the drug chosen (79) and line of therapy but incidence of severe nausea increased from <1% with monotherapy to 2–3% with combinations (1,2,5,7,9-12,80).
Management
The main differential diagnoses of diarrhoea includes GI infection and/or tumour-related symptoms (73). A blood test including C-reactive protein and albumin levels should be performed. A stool analyses for bacterial/viral pathogens, parasites and Clostridium difficile toxin should be also ruled out. An abdominal CT scan will establish the severity and extension of the GI disease. Finally, a colonoscopy with biopsy will confirm the diagnosis (56).
Grade 1 or 2 diarrhoea should be treated with oral loperamide and fluid and electrolyte supplementation (56). For toxicity beyond grade 2, treatment according Table 2. If no response, stop steroids and start with a single or two doses of infliximab (5 mg/kg) (74). Vedolizumab, integrin monoclonal antibody against the intestinal T lymphocytes, has shown preliminary efficacy as an infliximab alternative (81). Neither corticosteroids nor infliximab appear to affect the overall response rate and survival of patients treated with immunotherapy (76).
Renal toxicity
Renal dysfunction and urinary disorders are rare ir-AE’s (82). Most common Incidence is low grade 1–2 reported up to ~2–3% in nivolumab and pembrolizumab NSCLC-treated patients (1,2,9-11), and higher grades (≥3) are occasional (<1%) and reversible in most cases upon drug discontinuation and immune suppressive agents. As observed with other tumors, in NSCLC the incidence of renal ir-AE’s increases up to 8–11% with combinations of ICIs, ipilimumab or tremelimumab with PD-1/PD-L1, as well as the rates of severe G 3–4 renal toxicity (~3–5%) (5,32) (Table 1). Median time to onset of renal-selected events is highly variable according to literature (6 to 30 weeks) and median time to resolution is rapid as compared to other ir-AEs ranging from 3 to 6 weeks (83). In nivolumab second-line phase 3 trials (9,10), median time to onset of all grade renal ir-AE’s was from 6 to 10 weeks. Half of the patients with documented renal ir-AEs resolved toxicity and the majority did not require immune-modulating medication. Acute tubule-interstitial nephritis and glomerulonephritis characterized by a lymphocytic infiltrate are the most common ir-AE’s damages (84). Recently a case of glomerulonephritis mediated by anti-neutrophil cytoplasmic antibody has been described in a thymic epithelial tumor (TET) treated with pembrolizumab (85). However, few cases of renal documented parenchymal damage have been reported with ICIs in NSCLC trials probably due to the lack of histologic confirmation, being creatinine impairment the most commonly documented event. Treatment with ICIs is currently explored in combination with chemotherapy, such as in the phase 2 KEYNOTE-021 trial (7), which reported only 2 cases of acute kidney injury in the combination arm with 10% mild G 1–2 creatinine increase. However, it is difficult to discern in NSCLC patients, whether this toxicity was attributable to the ICI or to chemotherapy itself. Renal safety profile of combination of ICIs and chemotherapy will be also assessed in other ongoing phase III clinical trials (NCT02477826, NCT02578680, NCT02657434).
Management
Renal dysfunction must be suspected in patients presenting alterations in laboratory tests such as elevated serum creatinine, alteration in serum electrolytes (hypokalemia, hyponatremia) urinary sediment or proteinuria (55). For proper identification, a complete baseline assessment of renal function with biochemistry tests (sodium, potassium, creatinine and urea) must be performed before every infusion. Unlike endocrine and skin toxicities, renal toxicity and function recovers back rapidly with the introduction of proper hydration, immunosuppression and drug discontinuation (56). This is important as any delay in treating this complication could induce a permanent renal injury. Treatment are based according recommendations in Table 2 (56,83). Renal biopsy can be considered in grade 2 to 4, after discussion with the nephrologist, if clinical data is misleading with other common causes of renal impairment in NSCLC patients such as intravenous contrasts, concomitant medications or hypertension, although the level of evidence that supports this endorsement is low (56,83).
Liver toxicity
In a recent meta-analyses of phase II/III trials of cancer patients showed that CTLA-4 inhibitors unlike PD-1 inhibitors are associated with a higher risk of all- and high-grade hepatotoxicity (86). In NSCLC, incidence of all grades and grade ≥3 liver toxicity rates do not differ that much among different PD-1/PD-L1 inhibitors being 2–7.6% and ~1%, respectively (4,9-12,15,31). However, nivolumab in first-line setting (2) reported slightly higher all- and high-grade hepatic toxicity, 12% and 3%, respectively. No hepatotoxicity was reported with first-line pembrolizumab (1). Combination strategies [PD-(L)1 plus CTL4 inhibitor or chemotherapy] enhanced hepatic toxicity to 3–20% for all grades and up to 9% for grade ≥3 (5,7,32). In SCLC, combinations of ICIs compared to monotherapy also increase risk of any grade and grade ≥3-hepatic toxicity. In the phase I CheckMate 032 trial hepatotoxicity was slightly higher with nivolumab plus ipilimumab compared to nivolumab single agent (all grades, 12% vs. 6% and grade 3–4, 6% vs. 2%, respectively), with one treatment related death due to autoimmune hepatitis in the combination arm (26).
Immune-related hepatitis-like event normally involves transaminases elevation but cholestatic forms with increased bilirubin are uncommon (87). Recently, in an advanced NSCLC patients treated with nivolumab has been reported a novel form of cholangitic liver disease characterized by a high prevalence of T-cell infiltrate in the portal tract (88).
Hepatitis can occur in broad range of time and recovery, and as observed in kidney, usually occurs in 2–6 weeks (83). In NSCLC patients, median time to onset of hepatitis of 1.9 weeks and the majority of patients resolved toxicity within 8 weeks with appropriate treatment (10).
Management
Blood monitoring of gamma-glutamil-transpeptidase, alkaline phosphatase, as well as transaminases and bilirubin may be helpful to rule out liver toxicity and cholestatic liver disease as it commonly presents as asymptomatic. Other causes of viral infections such as Hepatitis A, B or C, autoimmune diseases and specific-drugs must be rule out for differential diagnose. Treatment recommendations are summarized in Table 2 (56,83). Treatment resumption is not considered in grade 4 toxicity and in grade 3 risk/benefit balance must be carefully discussed according to some guidelines (55,56). Liver biopsy might be deemed in refractory grade 3 or 4 liver events (56,83). Cases of successful treatment in refractory toxicities with mycophenolate mofetil 500–1,000 mg twice daily or tacrolimus and anti-thymocyte globulin (ATG) have been reported (89). Be advise that the use of infliximab is contraindicated for potential hepatotoxic associated side effects (56,83).
Endocrine toxicity
Over 12% of patients receiving ipilimumab, nivolumab, and/or pembrolizumab may develop an endocrine ir-AE (90). In a recent meta-analysis (38 studies, N=7,551 patients), the overall incidence of hypothyroidism was estimated to be 6.6%, reaching 13.2% with combination therapy, without differences in incidence between PD-1 and PD-L1 therapies. The overall incidence of hyperthyroidism was 2.9% and a statistically significant difference was observed among the classes of immunotherapy, with increased risk with anti-PD-1 than with PD-L1 inhibitors (OR, 5.36; 95% CI, 2.04–14.08; P=0.002) (91). Other endocrine ir-AEs, such as hypophysitis had increased incidence with combination therapy (6.4%) compared with monotherapy (3.2% with CTLA-4 inhibitors; 0.4% with PD-1 inhibitors; and less than 0.1%, PD-L1 inhibitors). However, despite its rarity, usually are severe toxicities (grade ≥3) (91).
In NSCLC, the incidence of endocrine ir-AEs has been described with the use of anti-PD-1/PD-L1 inhibitors (~1–10%) and is increased with the use of CTLA-4/ anti-PD-1/PDL-1 combination strategies (~11–23%) (92). In recurrent advanced SCLC (62) the incidence of any grade and grade 3–4 endocrine toxicity was also significantly increased in the combination cohort with respect nivolumab monotherapy (all grades, 8% vs. 21% and grade 3–4, 0% vs. 3%, respectively).
Thyroid dysfunction is the most common type of endocrine disorder in NSCLC with an incidence of ~1% to 9%, with uncommon grade 3 toxicity (1,2,4,9-11,15,31). Hypothyroidism is the most common event (~4–8%) whereas hyperthyroidism and thyroiditis (Hashimoto’s disease) are less frequently reported (~2–3%). Reversible myxedema cases on nivolumab have also been reported (93). Incidence of hypothyroidism increases to ~10% with combinations (5,32), however, despite this increase, the majority of these ir-AEs are easily manageable being the majority mild grade 1–2 (Table 1). Also, increased risk of endocrine ir-AE’s has been reported when ICI are combined with chemotherapy (7) (Table 1). Hypothyroidism is frequently preceded by an earlier and transient period of asymptomatic hyperthyroidism induced by an acute inflammation of the thyroid gland (94). Median time of onset is not well established, but has been reported to be ~4–6 weeks in nivolumab second line trials (9,10).
In NSCLC, close monitoring of thyroid function (blood tests for TSH, free T3, free T4 and anti-thyroid antibodies) increases diagnosis of thyroid dysfunction to 21% (94), incidence much higher to that observed in other series of NSCLC patients treated with anti-PD-1/PD-L1 monotherapy which might reflect the undervalued diagnose in clinical trials. Remarkably, OS was found significantly longer in those patients who developed thyroid dysfunction (median 40 vs. 14 months, HR 0.29; 95% CI 0.09–0.94; P=0.04) (94). Of note, the majority of patients with pre-existing hypothyroidism (94) do not require dose adjustments in thyroid hormone replacements, so medical records of hypothyroidism does not seem to preclude the use of immunotherapy supporting its safety use.
The incidence of hypophysitis in NSCLC patients treated with anti-PD-1/PD-L1 is <1% (1,2,9-11) and it does not seem to increase with the use of immunotherapy combinations (5,32) (Table 1). Immune-induced diabetes mellitus is also very uncommon event (<1%) and nor dose schedules of PD-1 inhibitors [pembrolizumab 2 mg/kg vs. 10 mg/kg (1,11)] neither the use of combinations strategies seem to increase its incidence [the only one case of G4 diabetes reported in the phase I nivolumab plus ipilimumab trial was not described as immune-related AE (5)].
Primary adrenal insufficiency is also a sporadic event (<1%) described with nivolumab, pembrolizumab and avelumab (10,11,15). However, unlike both aforementioned rare endocrine ir-AEs, namely hypophysitis and diabetes mellitus, a trend to a higher incidence (~12%) was observed in the higher cohort of nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks (5). No predictive markers of risk to develop endocrine toxicity have been identified so far. Eosinophilia has been suggested as a potential early indicator of adrenal insufficiency by ipilimumab before the onset of symptoms (95).
Recently a case of NSCLC patient with no medical records who displayed diabetic ketoacidosis after nivolumab treatment, demonstrated the presence in blood of islet cell diabetes autoantibodies before the initiation of nivolumab, suggesting the potential utility of detecting pre-existing autoantibodies to identify predisposed patients at higher risk of developing immune-mediated diabetes (96).
Management
High index of suspicion and periodic measurement of thyroid function tests (TSH, free T3 and free T4) are recommended (93), especially with the use of CTLA4 combinations (56). In case of PD-1/PDL-1 inhibitors treatment, thyroid functions tests monitoring before every infusion for the first 3 months and at least once a month thereafter (every second cycle in the case of biweekly administrations) is recommended (56).
Measurement of anti-thyroid peroxidase (anti-TPO), thyroglobulin or thyrotropin receptor antibodies might be only required in those cases in which autoimmune disease (Hashimoto’s or Grave’s disease) is suspected (56,83).
Treatment recommendations are summarized in Table 2. Use of steroids is not frequent. Substitutive treatment with thyroid hormone replacement (thyroxine 0.5–1.5 µg/kg) is normally sufficient to recover symptoms and is usually long lasting. In symptomatic hyperthyroidism, thyrotoxicosis, the use of beta-blockers is recommended whereas the use of carbimazole is exceptional and only recommended in cases with anti-TSH receptor antibodies (56).
The autoimmune hypophysitis can overt symptoms related to the pituitary-endocrine axis disruption, that is to say an adrenal, gonadal and thyroid hormonal deficiency, and only in rare cases can be associated to an inadequate antidiuretic hormone (ADH) secretion leading to a central diabetes insipidus (56,92). Symptoms and severity vary according to the hormone insufficiency induced and have been comprehensively reviewed elsewhere (92). Diagnose must be done under clinical suspicious by assessing a panel of blood tests for pituitary-axis (thyroid: TSH, T3, T4; also: ACTH, IGF-1, Prolactin, cortisol, LH, FSH and testosterone, estradiol in males and females, respectively) as well as a pituitary magnetic resonance imaging (MRI) scan to confirm diagnose and exclude hypophysis enlargement (56). Treatment consists on initial replacement of hormone deficiency with special advice for thyroid hormones. Steroids treatment dose are reported in Table 2. Steroids must continue once symptoms improve by tapering over 2–5 weeks to 5 mg along with proper hydrocortisone substitution (25–40 mg/day) (56,83). After recovery of toxicity, half of the patients will need permanent hormone replacement and the vast majority will be unable to recover from persistent adrenal insufficiency (56,92).
Although autoimmune DM associated with immunotherapy is an extremely rare event, some cases present as life-threatening ketoacidosis (96). Therefore, regular monitoring blood glucose levels are recommended to early identify the new onset of immune-mediated diabetes. Additionally, for plasma testing for islet cells (ICA) or Glutamic Acid Decarboxylase autoantibodies (GADA) as well as C-Peptide might be needed to differentiate type 1 from type 2 diabetes. Treatment with insulin is mandatory and the used of cortisone is not encouraged. As observed with other immune-mediated endocrine toxicities, treatment with immunotherapy can be resumed once symptoms are regulated with insulin requirements (56).
Less common toxicities
Cardiac toxicities
Cardiac events with immunotherapy remain an uncommon toxicity <1%, but includes a wide spectrum of toxicities such as myocarditis, pericarditis, arrhythmias, cardiomyopathy. The incidence of cardiac toxicity is increased with combination of ICIs including an anti-CTLA-4 (56,97). Also, patients with history of malignant involvement of pleural spaces could have higher risk of onset pleural or pericardial effusion during treatment (98-100). The incidence of myocarditis among NSCLC patients in phase III clinical trials is 0.15% (101). Of note, among thymic carcinoma patients treated with pembrolizumab, the incidence of myocarditis was 5% and all patients required a pacemaker (27). In recurrent SCLC, cardiomyopathy grade 4 was reported in 2% of cases with the combination of nivolumab and ipilimumab without any case in nivolumab monotherapy arm (62). It is unknown whether previous thoracic radiotherapy or anthracycline treatment in thymic carcinoma patients might explain the increased incidence of this toxicity. In a phase II study combining pembrolizumab and platinum pemetrexed, one patient (2%) presented myocardial infarction (7). However, it is difficult to know whether this toxicity is attributable to the ICIs, to the platinum-agent or combination. Based on the low reported frequencies, for patients of a history of cardiovascular event a baseline electrocardiogram would be appropriate (101).
Management
Early consultation with a cardiologist is recommended in case of suspicion. Administration of steroids and other immunosuppressive agents in case of worsening and no responses to steroids is recommended (56) (Table 2).
Neurologic events
Neurological adverse events associated with ICIs have a diverse phenotype, and peripheral ir-AE’s outweighed central ones in anti-PD1 cases (2.2:1 ratio) versus anti-CTLA-4 (1.7:1 ratio). Interestingly, 75% of patients with neurological ir-AE’s do not report other concurrent systemic ir-AEs (102). Although rare, neurological ir-AE’s incidence is increasing as anti-PD-1 therapies expand use to different cancers. The time of onset is unpredictable, and evolution may be rapid and life threatening. A very recently analysis of 59 trials, with 9,208 patients reported an incidence of 3.8% in patients treated with CTLA-4 inhibitors, 6.1% with PD-1 inhibitors and 12% with the combination of both drugs; the time to onset was from 6 to 13 weeks (56), but some cases of delayed onset, between 31 and 76 weeks after therapy initiation, have also been reported (102). In other recent retrospective review, among 347 patients with several tumours treated with pembrolizumab or nivolumab, 10 (2.9%) developed subacute onset of neurological complications (2 of them adenocarcinoma NSCLC patients). Neurological complications occurred after a median of 5.5 (range, 1–20) cycles of anti-PD-1 inhibitors (103). Multiple ir-AE’s have been reported with PD-1 inhibitors and CTLA-4 inhibitors, as monotherapy or in combination; but most of them has been mainly linked to ipilimumab in melanoma patients (9,10,97,102,104-106). These ir-AE’s include polyneuropathy, facial nerve palsy, demyelination, myasthenia gravis, Guillain-Barre syndrome, posterior reversible leukoencephalopathy, transverse myelitis, enteric neuropathy, encephalitis and aseptic meningitis (56). With no specific symptomatology, and a large variety of neurologic syndromes and severity, these events are particularly difficult to diagnose, emphasizing the differential diagnosis with tumor progression, neurologic primary event, infection or metabolic event (9,10,97,102,104-106).
In advanced melanoma patients treated with nivolumab with or without ipilimumab from 12 clinical trials, 35 patients (1%) presented with 43 neurologic ir-serious-AE’s. The median time to onset was 45 days (range, 1–170) (107). The median time for ir-AE’s resolution is 1 month (range, 2–809+ days). In 75% of cases symptomatology improves and resolves after treatment discontinuation (102,107). Among NSCLC patients, neurological ir-AE’s have not been reported. However, peripheral neuropathy could be misreported as consequence of chronic toxicity linked to previous platinum-based chemotherapy. Most of the reported ir-AE’s in NSCLC patients date is based on a very few clinical cases, including very serious cases of encephalopathy and myasthenia gravis-like, among others linked to pembrolizumab and nivolumab, respectively (106,108).
Management
According to the severity of these events, the imaging and complementary examination (nerve conduction studies, electroencephalogram and or a lumbar puncture) should be performed. If the ir-AE is considered severe, a consultation with a neurologist is required, and higher dose of systemic corticosteroids can be also recommended. The treatment with intravenous immunoglobulin and plasmapheresis could be considered. No specific surveillance is recommended for neurologic disorders beyond standard physical examination at clinic visits (56).
Pancreatic events
Asymptomatic elevations of lipase and/or amylase are the most common pancreatic ir-AE’s reported, not requiring generally immunosuppressive therapy (82).
In NSCLC patients, the elevation of amylase levels has been reported in CTLA-4 and/or PD-1/PD-L1 blockade in 0.6–3% (1,5,9,11,80); however, in the majority of patients did not meet diagnostic criteria of pancreatitis. No pancreatic toxicity was observed with atezolizumab (12) or nivolumab in non-squamous histology (10).
Management
Routine monitoring of pancreatic enzymes is not recommended and any therapy is recommended for minor asymptomatic elevations in these parameters (56). Globally, if a patient presents symptoms of pancreatitis, amylase and lipase should be measured, followed by imaging studies (109). The systemic corticosteroids can be recommended in serious pancreatic events according Table 2.
Hematological events
The hematological ir-AE’s are a very uncommon event. However, very serious cases of lethal aplastic anemia, autoimmune haemolytic anaemia or immune thrombocytopenic purpura among others, have been reported (56). A monocenter registry of immune-toxicity recently reported that among 908 patients treated with ICIs, 21 patients (2.3%) experienced systemic ir-AE’s, being the most frequent hematologic ir-AE the autoimmune cytopenia (thrombocytopenia, 2 patients, 0.2%) (34).
Management
The appropriate strategy to treat the severe hematological AE’s has not been yet established. The consultation with an hematologist is strongly recommended and the systemic corticosteroids and additionally other immunosuppressive drugs should be carried out, according to the severity of each event (56).
Eye events
The toxicity immune-related of the eyes occur in lower than 1% of the patients treated with ICIs. In general, the eye ir-AE’s are divided in: ocular inflammation (peripheral ulcerative keratitis, uveitis and Vogt-Koyanagi-Harada syndrome, orbital inflammation, including thyroid-associated orbitopathy and idiopathic orbital inflammation), and retinal and choroid disease (choroid neovascularization) (56).
Management
The therapy depends on the severity of each event. Topical corticosteroids are usually recommended if anterior chamber inflammation, and systemic corticosteroid for severe ocular or orbital inflammation. Intravitreal anti-vascular endothelial growth factor (VEGF) is indicated for choroid neovascularization (56).
Conclusions
Management of side effects related to ICIs among NSCLC patients is extremely important, as these therapies have changed treatment landscape of advanced NSCLC and probably will be incorporated in other settings of the disease. Based on lack of expertise of clinicians and the broader spectrum of uncommon toxicities, a close collaboration and multidisciplinary approach between oncologists and other specialists is strongly recommended to provide the best support and guidance for treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Jerusalem G, Chen F, Spigel D, et al. JAVELIN Solid Tumor: Safety and Clinical Activity of Avelumab (Anti-PD-L1) as First-Line Treatment in Patients with Advanced NSCLC. J Thorac Oncol 2017;12:S252. [Crossref]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol 2018;4:98-101. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Govindan R, Szczesna A, Ahn MJ, et al. Phase III Trial of Ipilimumab Combined With Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3449-57. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. [Crossref] [PubMed]
- Prasad V, Kaestner V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol 2017;44:132-5. [Crossref] [PubMed]
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Kindler H, Karrison T, Carol Tan YH, et al. OA13.02 Phase II Trial of Pembrolizumab in Patients with Malignant Mesothelioma (MM): Interim Analysis. J Thorac Oncol 2017;12:S293-4. [Crossref]
- Quispel-Janssen J, Zago G, Schouten R, et al. A Phase II Study of Nivolumab in Malignant Pleural Mesothelioma (NivoMes): with Translational Research (TR) Biopies. J Thorac Oncol 2017;12:S292-3. [Crossref]
- Scherpereel A, Mazieres J, Greillier L, et al. Second- or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Results of the IFCT-1501 MAPS2 randomized phase II trial. J Clin Oncol 2017;35: abstr LBA8507.
- Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34:8503.
- Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18:1261-73. [Crossref] [PubMed]
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823-9. [Crossref] [PubMed]
- Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts). J Clin Oncol 2017;35:8504.
- Sequist LV, Chiang A, Gilbert J, et al. Clinical activity, safety and predictive biomarkers results from a phase Ia atezolizumab (atezo) trial in extensive-stage small cell lung cancer (ES-SCLC). Ann Oncol 2016;27:493-6. [Crossref]
- Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35:8503.
- Giaccone G, Thompson J, McGuire C, et al. Pembrolizumab in patients with recurrent thymic carcinoma: Results of a phase II study. J Clin Oncol 2017;35:8573.
- Cho J, Ahn MJ, Yoo KH, et al. A phase II study of pembrolizumab for patients with previously treated advanced thymic epithelial tumor. J Clin Oncol 2017;35:8521.
- Heery CR, O'Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18:587-98. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299-308. [Crossref] [PubMed]
- Barlesi F, Steins M, Horn L, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol 2016;27:1215PD.
- Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34-44. [Crossref] [PubMed]
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790-801. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Auclin E, et al. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: Does age really matter? Cancer Treat Rev 2017;60:60-8. [Crossref] [PubMed]
- Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155-66. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Ferrara R, Caramella C, Texier M, et al. Hyperprogressive disease (HPD) is frequent in non-small cell lung cancer (NSCLC) patients (pts) treated with anti PD1/PD-L1 monoclonal antibodies (IO). Ann Oncol 2017;28:mdx 380.009.
- Nishijima TF, Muss HB, Shachar SS, et al. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat Rev 2016;45:30-7. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Popat S, Ardizzoni A, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: Results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged ≥70 years and with poor performance status. Ann Oncol 2017;28:v460-96. [Crossref]
- Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers. J Clin Oncol 2016;34:10010.
- Helissey C, Vicier C, Champiat S. The development of immunotherapy in older adults: New treatments, new toxicities? J Geriatr Oncol 2016;7:325-33. [Crossref] [PubMed]
- Leonardi GC, Gainor JF, Azimi RS, et al. Use of PD-1 pathway inhibitors among patients with non-small cell lung cancer (NSCLC) and preexisting autoimmune disorders. J Clin Oncol 2017;35:9081.
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377-85. [Crossref] [PubMed]
- Santini FC, Rizvi H, Wilkins O, et al. Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. J Clin Oncol 2017;35:9012.
- Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol 2017;35:3807-14. [Crossref] [PubMed]
- Owen DH, Wei L, Villalona-Calero MA, et al. Impact of immune-related adverse events (irAE) on overall survival (OS) in patients treated with immunotherapy for non-small cell lung cancer (NSCLC). J Clin Oncol 2017;35:9080.
- Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
- Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol 2017;28:2002-8. [Crossref] [PubMed]
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer 2017;5:43. [Crossref] [PubMed]
- Goldstein DA, Gordon N, Davidescu M, et al. A Phamacoeconomic Analysis of Personalized Dosing vs Fixed Dosing of Pembrolizumab in Firstline PD-L1-Positive Non-Small Cell Lung Cancer. J Natl Cancer Inst 2017.109. [PubMed]
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559-74. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22:6051-60. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref] [PubMed]
- Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. [Crossref] [PubMed]
- Fujimoto D, Morimoto T, Ito J, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017;111:1-5. [Crossref] [PubMed]
- Nishino M, Hatabu H, Hodi FS, et al. Drug-Related Pneumonitis in the Era of Precision Cancer Therapy. J Clin Oncol 2017.1-12.
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71:161-9. [Crossref] [PubMed]
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 2016;60:12-25. [Crossref] [PubMed]
- Tetzlaff MT, Nagarajan P, Chon S, et al. Lichenoid Dermatologic Toxicity From Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. Am J Dermatopathol 2017;39:121-9. [Crossref] [PubMed]
- Russo I, Sacco G, Frega S, et al. Immunotherapy-related skin toxicity: bullous pemphigoid in a lung adenocarcinoma patient treated with the anti-PDL1 antibody atezolizumab. Eur J Dermatol 2017;27:205-8. [PubMed]
- Uemura M, Faisal F, Haymaker C, et al. A case report of Grover's disease from immunotherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J Immunother Cancer 2016;4:55. Erratum in: J Immunother Cancer 2017;5:7. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283-9. [Crossref] [PubMed]
- Lord JD, Hackman RC, Moklebust A, et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci 2010;55:1396-405. [Crossref] [PubMed]
- Arriola E, Wheater M, Karydis I, et al. Infliximab for IPILIMUMAB-Related Colitis-Letter. Clin Cancer Res 2015;21:5642-3. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Shi Y, Lin P, Ho EY, et al. Nivolumab-associated nausea and vomiting as an immune adverse event. Eur J Cancer 2017;84:367-9. [Crossref] [PubMed]
- Pillai RN, Behera M, Owonikoko TK, et al. Evaluation of toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:9035.
- Gulley JL, Spigel D, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol 2015;33:8034.
- Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017;66:581-92. [Crossref] [PubMed]
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190-209. [Crossref] [PubMed]
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016;45:7-18. [Crossref] [PubMed]
- Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638-47. [Crossref] [PubMed]
- Heo MH, Kim HK, Lee H, et al. Antineutrophil Cytoplasmic Antibody-Associated Rapid Progressive Glomerulonephritis after Pembrolizumab Treatment in Thymic Epithelial Tumor: A Case Report. J Thorac Oncol 2017;12:e103-5. [Crossref] [PubMed]
- Wang W, Lie P, Guo M, et al. Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis of published data. Int J Cancer 2017;141:1018-28. [Crossref] [PubMed]
- Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol 2015;39:1075-84. [Crossref] [PubMed]
- Gelsomino F, Vitale G, D’Errico A, et al. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol 2017;28:671-2. [PubMed]
- Ahmed T, Pandey R, Shah B, et al. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep 2015;2015. [Crossref] [PubMed]
- Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf) 2018;88:327-32. [Crossref] [PubMed]
- Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:173-82. [Crossref] [PubMed]
- Rossi E, Sgambato A, De Chiara G, et al. Endocrinopathies induced by immune-checkpoint inhibitors in advanced non-small cell lung cancer. Expert Rev Clin Pharmacol 2016;9:419-28. [Crossref] [PubMed]
- Khan U, Rizvi H, Sano D, et al. Nivolumab induced myxedema crisis. J Immunother Cancer 2017;5:13. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Godwin JL, Jaggi S, Sirisena I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 2017;5:40. [Crossref] [PubMed]
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210-25. [Crossref] [PubMed]
- Kolla BC, Patel MR. Recurrent pleural effusions and cardiac tamponade as possible manifestations of pseudoprogression associated with nivolumab therapy- a report of two cases. J Immunother Cancer 2016;4:80. [Crossref] [PubMed]
- Kushnir I, Wolf I. Nivolumab-Induced Pericardial Tamponade: A Case Report and Discussion. Cardiology 2017;136:49-51. [Crossref] [PubMed]
- Nesfeder J, Elsensohn AN, Thind M, et al. Pericardial effusion with tamponade physiology induced by nivolumab. Int J Cardiol 2016;222:613-4. [Crossref] [PubMed]
- Ederhy S, Voisin A-L, Champiat S. Myocarditis with Immune Checkpoint Blockade. N Engl J Med 2017;376:290-1. [Crossref] [PubMed]
- Daher A, Tummala S. The Spectrum of Neurological Adverse Events from Immune Checkpoint Blockade: A Comprehensive Review of Literature. Neurology 2017;88:174.
- Kao JC, Liao B, Markovic SN, et al. Neurological Complications Associated With Anti-Programmed Death 1 (PD-1) Antibodies. JAMA Neurol 2017;74:1216-22. [Crossref] [PubMed]
- Makarious D, Horwood K, Coward JIG. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 2017;82:128-36. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [Crossref] [PubMed]
- Chen YH, Liu FC, Hsu CH, et al. Nivolumab-induced myasthenia gravis in a patient with squamous cell lung carcinoma: Case report. Medicine (Baltimore) 2017;96:e7350. [Crossref] [PubMed]
- Larkin J, Chmielowski B, Lao CD, et al. Neurologic Serious Adverse Events Associated with Nivolumab Plus Ipilimumab or Nivolumab Alone in Advanced Melanoma, Including a Case Series of Encephalitis. Oncologist 2017;22:709-18. [Crossref] [PubMed]
- Feng S, Coward J, McCaffrey E, et al. Pembrolizumab-Induced Encephalopathy: A Review of Neurological Toxicities with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1626-35. [Crossref] [PubMed]
- O'Kane GM, Labbé C, Doherty MK, et al. Monitoring and Management of Immune-Related Adverse Events Associated With Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 2017;22:70-80. [Crossref] [PubMed]


