New horizons from immunotherapy in malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is a fatal disease, mostly related to previous asbestos exposure (1). Its incidence is on the rise in the industrialized countries (2) and will reach its peak in the second to third decade of this century (3). The prognosis of MPM remains dismal because it is often diagnosed in an advanced stage of disease. Currently, an antifolate and platinum combination regimen represents the only established treatment for patients not amenable to curative surgery; however, this approach is largely unsatisfactory due to its limited impact on long-term survival of patients (4). Recently, a large randomized French study (MAPS) has shown that the addition of bevacizumab to cisplatin and pemetrexed results in an added benefit of 2.7 months in overall survival (OS) compared to standard therapy (5); however, this regimen is not yet considered a new standard of care in most countries. For patients who failed front-line chemotherapy the prognosis is even more dismal, as no standard second-line treatment has been yet defined (6). Among different therapeutic strategies largely investigated in MPM, immunotherapy represents a very promising approach (7). Indeed, spontaneous tumor-specific immune responses have been reported in MPM patients, and a better prognosis in patients with a high number of tumor-infiltrating immune cells has been demonstrated (8,9). In light of this evidence, a variety of clinical studies in the past explored the activity of different immunotherapeutic agents, in particular interferon- or interleukin-2-based regimens; unfortunately, these agents demonstrated limited efficacy or they were burdened with severe toxicity (10-13). A limited knowledge of multiple mechanisms of immune suppression operated by tumor cells, which include high levels of regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages in the tumor microenvironment, as well as non-appropriate methods for evaluating tumor response in the course of immunotherapy, could contribute, at least in part, to the failure of previous anti-tumor immunotherapeutic strategies (14).
In the recent years, a deeper understanding of the dynamic associations between pro-tumorigenic and anti-tumorigenic components of the MPM microenvironment and the interactions between tumor cells with host immune system have sparked new hopes to cure this disease with immunotherapy (15,16). Along this line, a number of immunotherapeutic clinical trials, aimed at activating the host’s immune system or overcoming components of the immunosuppressive tumor microenvironment, have been activated (17). Among these, the two main approaches of immunotherapy currently under investigation in MPM are focused on the targeting of immune-checkpoint inhibitors and mesothelin.
Immune checkpoint inhibitors
The remarkable progress in the clinical application of anti-tumor immunotherapy is mostly due to the development of therapeutic mAb (so-called immunomodulating mAb) targeting regulatory immune-checkpoints; these molecules are physiologically expressed on immune cells and play a crucial role in maintaining immune homeostasis and ensuring self-tolerance by mediating signals that attenuate excessive immune activation. Immunomodulating mAb restore and unleash anti-tumor activity of cytotoxic T cells by blocking inhibitor molecules on T cells or their ligand expressed on antigen presenting cells (APC) or tumor cells (18,19). This novel strategy has demonstrated its feasibility and efficacy in significantly prolonging long-term survival of patients with different malignancies in a large number of clinical studies, thus opening a new era in the history of cancer treatment.
Anti-cytotoxic T lymphocyte (CTLA)-4 mAb
CTLA-4 is a glycoprotein, member of the CD28 family receptors, inducibly expressed on the surface of activated CD4+ and CD8+ T cells, and constitutively expressed on regulatory T cells (19). CTLA-4 competes with CD28 costimulator receptor for the binding to ligand B7 (CD80 or CD86) expressed on APC; as CTLA-4 binds with higher affinity than CD28, it reduces CD28-dependent costimulation, and mediates direct inhibitory effects on the MHC-TCR pathway (19). Anti-CTLA-4 mAb by blocking CTLA-4 prevents its binding to B7, thus allowing T cells activation. Anti-CTLA-4 antibodies represent the prototype of this growing family of immunomodulating mAb targeting immune-checkpoints (19). Two anti-CTLA-4 mAbs are in clinical development: ipilimumab and tremelimumab. Ipilimumab represents the first of its class to demonstrate its ability to significantly improve the survival of metastatic melanoma patients (20), thus broadening its therapeutic exploration and prompting the clinical development of additional checkpoint blocking mAb in different tumor types, including malignant mesothelioma (MM) (21).
MESOT-TREM-2008 is the first study that explored the activity and safety of anti-CTLA-4 mAb in MM patients (22). In this phase II study, tremelimumab was administered at 15 mg/kg intravenous (IV) every 90 days in 29 second-line MM patients. Despite a low objective response rate (ORR) that was 7%, a long-lasting disease control and 2-year survival rate were observed in 31%, and 36% of MM patients, respectively (22). Additionally, grade 3–4 treatment-related side effects were observed in a minority of patients (22). These promising results were corroborated in the phase II MESOT-TREM-2012 study that investigated the activity and safety of tremelimumab in 29 additional second-line MM patients (23). In this second study, tremelimumab was given at an intensified dosing schedule of 10 mg/kg IV every 4 weeks (wks) for 6 doses, followed by administration of tremelimumab every 12 wks, based on previous pharmacokinetic data in metastatic melanoma patients (24). Four patients (14%) achieved an immune-related (ir)-ORR, thus the study reached its primary endpoint; among secondary endpoints explored, the ir-disease control rate (DCR) was 52%, the median duration of DCR was 10.9 months, and the median OS was 11.3 months; treatment was overall well tolerated, with grade 3–4 treatment-related toxicity observed in 7% of patients (23). These promising results contributed to the activation of a large, placebo controlled phase IIb study (DETERMINE); in this pivotal study tremelimumab was investigated in 571 second/third line MM patients at the same intensified schedule of administration utilized in the MESOT-TREM-2012 study (25). Unfortunately, the study did not show a superiority of tremelimumab for the primary endpoint of OS compared to placebo (25). Despite the failure of the study, this antibody class has had the merit of paving the way for the exploration of more effective immune checkpoint inhibitors, particularly those directed against programmed cell death protein (PD)-1 or its main ligand PD-L1 in this disease.
Anti-PD-1/PD-L1 mAb
PD-1 is a trans-membrane inhibitory immune-receptor, member of the B7-CD28 family, expressed on activated T, B, and natural killer cells (26). It binds to PD-L1 or PD-L2 that are expressed on stromal and tumor cells; these interactions lead to a reduction of cytotoxic T cells, release of cytokines, proliferation, and finally to a depletion of T cells (26,27). Blocking PD-1 or PD-L1 by immunomodulating mAb, de-represses T cell activation, unleashing a clinical immune response towards the tumor (27). A growing number of anti-PD1/PD-L1 mAb has been recently approved in a variety of solid and hematological malignancies (28) thus prompting their investigation in additional tumor types including MPM.
The expression of PD-L1 has been reported in up to 60% of MPM samples in different series, with a higher rate in sarcomatoid histotype, and it has been associated to a poor prognosis (29-32). A growing number of phase I/II clinical studies with drugs targeting PD-1/PD-L1 axis are currently ongoing. In the phase Ib multi-cohort KEYNOTE-028 study, the anti-PD-1 pembrolizumab was investigated in PD-L1 positive pretreated MPM patients at a dose of 10 mg/kg every 2 wks (33). Five (20%) out 25 patients achieved a partial response (PR), and 13 (52%) a stable disease (SD); noteworthy, responses were durable with an average response duration of 12.0 months. Interestingly, the median progression free survival (PFS) was 5.4 months and the median OS was 18.0 months (33). The encouraging results observed in this first study prompted a rapid and large development of agents directed against the PD-1/PD-L1 axis in MPM. In a single-center phase II study, still ongoing at the University of Chicago (34), pembrolizumab was given in MPM and peritoneal MM patients at 200 mg every 3 wks; eligible patients were progressed to 1 or 2 prior regimens, and were unselected for PD-L1 status. Initial results reported an ORR of 21% and a DCR of 59%; median PFS was 6.2 months, and median OS was 11.9 months. Translational studies did not demonstrate a significant correlation between responses and PD-L1 expression or interferon-gamma gene expression profile (34). The activity of the anti-PD-1 nivolumab was investigated in the ongoing phase II NIVO-MES trial; 34 relapsed MPM patients received nivolumab at 3 mg/kg every 2 wks; preliminary data reported an ORR of 15%, regardless the PD-L1 expression on tumor cells, and a SD of 35%; median PFS was 3.6 months (35). In the phase II MERIT study, nivolumab was investigated at a flat dose of 240 mg IV every 2 wks in 34 second or third line MPM; results showed that 29.4% and 67.6% of patients reached an ORR and a DCR, respectively; in addition the PFS was 6.1 months and the median OS not reached at the time of that analysis (36). The role of nivolumab in pretreated MPM or peritoneal MM patients is currently being investigated in the randomized phase III double-blind, placebo-controlled CONFIRM study (37). In this study, patients progressed to at least two prior lines of chemotherapy are randomized in a 2:1 ratio to receive nivolumab at a flat dose of 240 mg or placebo. The trial has been recently opened in the United Kingdom, and will enroll 336 patients (37). In the phase Ib multicohort JAVELIN study, the anti-PD-L1 avelumab at the dose of 10 mg/kg every 2 wks was investigated in 53 MM patients progressing to at least one prior platinum/pemetrexed regimen (38). Patients were heavily pretreated, with a median of two prior treatments. A durable PR was observed in 5 (9%) patients; and an overall DCR was observed in 56% of patients. Median PFS was 17.1 wks, and the 24-week PFS was 38.4%. The most common treatment-related toxicity included fatigue, fever, infusion-related reactions, and dermatological side effects, similarly observed in trials with anti-PD-1 mAb (38).
Overall, these results indicate that targeting the PD-1/PD-L1 axis in MPM appears promising; however, these results have to be considered with caution because they are still very preliminary, and most of these trials are still ongoing. Therefore, several important issues regarding the role of these agents in MPM need to be further explored. Major efforts are currently directed to identify predictors of response to immune-checkpoint inhibitors, such as tumor molecular features or characterization of immune infiltrates in the tumor microenvironment, for a better patient selection for this therapeutic approach.
Combination strategy with immune-checkpoint blocking mAb
Though promising, the clinical benefit with PD-1/PD-L1 inhibitors is achieved by a limited proportion of MPM patients; to extend their benefit to a large population and to overcome primary or acquired immune-resistance observed in the majority of patients, current efforts are directed towards combined regimens.
Blocking of CTLA-4 could represent an optimal partner for combination regimen with PD-1/PD-L1 inhibitors; indeed these molecules act in two distinct phases of T cell activation; therefore, an additive or synergistic effect may be supposed by blocking these pathways. Consistently, evidence has shown a higher efficacy of nivolumab in combination with ipilimumab compared to nivolumab or ipilimumab alone in metastatic melanoma patients (39,40); along this line, a growing number of phase III clinical studies investigating the efficacy of combining CTLA-4 blockade with PD-1/PD-L1 blocking mAb are currently under investigation in different malignancies.
In the phase II NIBIT-MESO-1 study, the therapeutic potential activity of tremelimumab in combination with anti-PD-L1 mAb durvalumab was investigated in MPM and peritoneal MM patients (41). Forty patients received treatment with tremelimumab at a dose of 1 mg/Kg IV every 4 wks in combination with the anti-PD-L1 durvalumab at 20 mg/Kg IV every 4 wks for 4 doses during the induction phase, followed by durvalumab in monotherapy for additional 9 doses, in a maintenance phase. Primary endpoint of the study was to assess the ir-ORR in the study population; among secondary, were ir-DCR, ir-PFS, OS, and safety. The study is still ongoing but not recruiting. Safety analysis, was reported at ASCO meeting 2017, and demonstrated the tolerability of this combination regimen; indeed, most patients experienced mild or moderated ir toxicity (67.5%), and grade 3–4 treatment-related side effects were observed in 17.5% of patients; treatment-related toxicity was overall reversible according to protocol guidelines (41). Final efficacy analysis of NIBIT-MESO-1 study is currently ongoing.
In the phase II, randomized, non-comparative MAPS-2 study, nivolumab was investigated alone or in combination with ipilimumab in second or third line MPM patients. Final results have been recently shown at ASCO meeting 2017, and reported at week 12, a DCR of 44% or 50% with nivolumab alone or in combination with ipilimumab, respectively, thus the study reached its primary endpoint; among secondary endpoints explored, the median OS was 10.4 months with nivolumab alone, while it was not yet reached in the combo arm. Seventeen percent of patients experienced severe treatment-related side effects (42).
Several combination studies are currently ongoing, among these, the large, randomized, phase III Checkmate-743 study (NCT02899299) is currently investigating the efficacy of nivolumab in combination with ipilimumab in comparison to standard chemotherapy in first-line MPM patients; the Italian-Canadian phase III study is evaluating the efficacy of pembrolizumab alone or in combination with platinum-based regimen compared to chemotherapy alone in first-line MPM patients (NCT02784171). Additionally, a phase II study is exploring the immunological activity of durvalumab alone or in combination with tremelimumab in surgically resectable MPM (NCT02592551). Novel immune checkpoints are currently in early phase of clinical exploration in different tumor types; among these, in the phase I INDUCE-I study (NCT02723955), the safety and activity of GSK3359609 targeting the inducible T cell co-stimulator (ICOS), alone or in combination with pembrolizumab, is under investigation in selected advanced solid tumors including MPM. In Table 1 the main ongoing trials with immune checkpoints blockade utilized alone or in combination with different agents are reported.
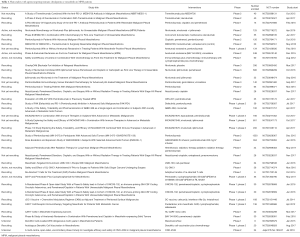
Full table
Immune-targeting of mesothelin
Mesothelin is a cell-surface glycoprotein, highly expressed in many solid tumors, including mesothelioma, with limited expression in normal tissues (43); therefore, it represents an optimal therapeutic target. Along this line, a variety of compounds for targeting of mesothelin with different mechanism of action are currently at various phases of clinical development; they mostly include chimeric mAb amatuximab, recombinant immunotoxins (SS1P, RG7787/LMB-100), antibody-drug conjugates (such as anetumab ravtansine), and chimeric antigen receptor (CAR)-T cells (44).
- Amatuximab is a mouse-human chimeric anti-mesothelin mAb; in a phase II study, it was investigated in combination with cisplatin and pemetrexed in MPM patients with promising results in median OS that was 14.8 months (45); therefore a randomized, phase II trial was launched but prematurely closed due to low accrual (46).
- Anetumab-ravtansine is an antibody-drug conjugate; after its binding with mesothelin expressed on tumor cells, the antibody-drug conjugate is internalized and releases the cytotoxic agent ravtansine (47). In a small phase Ib study, anetumab-ravtansine showed a response rate in pretreated MPM patients of 50%, and a DCR of 90%; unfortunately, in the subsequent randomized phase II study (47), this compound failed to demonstrate an improvement in survival in comparison to vinorelbine in second-line MPM patients (48,49).
- Recombinant immunotoxins: SS1P (anti-mesothelin dsFv-PE38) consists of a murine anti-mesothelin disulfide-stabilized single-chain Fv fragment (targeting moiety) linked to PE38 (effector moiety), the protein-synthesis-inhibiting domain of Pseudomonas exotoxin A (50). In phase I studies, SS1P generated neutralizing antibodies to the pseudomonas endotoxin (PE) (51,52); therefore, in a subsequent study, pentostatin and cyclophosphamide were given before the administration of SS1P to deplete T and B lymphocyte, thus delaying the development of neutralizing antibodies; initial signs of activity were observed in 3/10 patients treated in a phase I study (53). To minimize immunogenicity, Hollevoet et al. re-engineered the targeting moiety from mouse dsFv to humanized Fab and de-immunized the effector moiety PE to generate a new immunotoxin, called RG7787/LMB-100 (54). A phase I study to assess the maximum tolerated dose and the immunogenicity of RG778 is currently under way in MPM patients (NCT02798536).
- Mesothelin CARs: adoptive T cell therapy using engineered T cells directed towards tumor antigens (CAR-T) is another promising approach that has shown impressive clinical outcomes in leukemia, and it is now being investigated in solid malignancies (55). Mesothelin is an especially appealing target for this approach since it is overexpressed in the majority of MPM, and several preclinical and clinical studies have found that is involved in tumorigenesis, as well as being associated with tumor aggressiveness (56). Data generated in CAR-T cells, mainly directed against mesothelin in MPM patients, demonstrated early signs of clinical activity and T cell reactivity towards the tumor. Mesothelin CARs are currently being investigated in multiple phase I clinical trials (NCT02414269, NCT01583686, NCT02580747, NCT02159716, and NCT01355965). Further adaptations of the CAR-T cell strategy, including intrapleural delivery approaches, are under investigation to increase tumor infiltration and decrease treatment-related side effects (57).
Other immunotherapeutic approaches
Additional immunotherapeutic strategies, including vaccines (such as CRS-207, a Listeria monocytogenes expressing human mesothelin), intrapleural administration of an adenovirus expressing interferon alpha (Ad.IFN-α), vaccination with a Wilms’ tumor-1 (WT-1) peptide analogue, dendritic cell vaccine, are currently under investigation in early phases of clinical studies (44). Table 1 reports the currently ongoing main trials, investigating the activity and safety of these therapeutic approaches.
Future directions/perspectives
Much has to be gained in the therapeutic scenario of MPM: the heterogeneity and the relatively low incidence of this disease, together with the difficult radiological evaluation of tumor response in MPM patients, particularly in the course of treatment with immunotherapeutic agents, pose barriers to developing more effective systemic therapies. However, in the last decade, a significant growth in the knowledge of mesothelioma immune-biology has translated into the development of a variety of novel immunotherapeutic agents that are beginning to show clinical potential in MPM patients. Targeting immune-checkpoint inhibitors and mesothelin, including combinations of these novel agents, appear to be among the most encouraging of the emerging therapeutic approaches.
Acknowledgements
Funding: This work was supported by unrestricted grants from Associazione Italiana per la Ricerca sul Cancro (IG15373, 2014).
Footnote
Conflicts of Interest: L Calabrò served on Advisory Board of Bristol Myers Squibb; M Maio served on Advisory Boards of Bristol Myers Squibb, Roche-Genentech, AstraZeneca-MedImmune. The other author has no conflicts of interest to declare.
References
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24,724A-724C.
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379-87. [Crossref] [PubMed]
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491-6. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Buikhuisen WA, Hiddinga BI, Baas P, et al. Second-line therapy in malignant pleural mesothelioma. Lung Cancer 2015;89:223-31. [Crossref] [PubMed]
- Thapa B, Watkins DN, John T. Immunotherapy for malignant mesothelioma: Reality check. Expert Rev Anticancer Ther 2016;6:1-10. [PubMed]
- Yamada N, Otzumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother 2010;59:1543-9. [Crossref] [PubMed]
- Robinson C, Robinson BW, Lake RA. Sera from patients with malignant mesothelioma can contain autoantibodies. Lung Cancer 1998;20:175-84. [Crossref] [PubMed]
- Ho M, Hassan R, Zhang J, et al. Humoral immune response to mesothelina in mesothelioma and ovarian cancer patients. Clin Cancer Res 2005;11:3814-20. [Crossref] [PubMed]
- Castagneto B, Zai S, Mutti L. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: results of a phase II on 31 consecutive patients. Lung Cancer 2001;31:303-10. [Crossref] [PubMed]
- Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008;44:46-53. [Crossref] [PubMed]
- Powell A, Creaney J, Broomfield S, et al. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer 2006;52:189-97. [Crossref] [PubMed]
- Cornelissen R, Lievense LA, Maat AP, et al. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS One 2014;9:e106742. [Crossref] [PubMed]
- Thapa B, Salcedo A, Lin X, et al. The immune microenvironment, genome –wide copy number aberrations and survival in mesothelioma. J Thorac Oncol 2017;12:850-9. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Menon S, Shin S, Dy G. Advances in cancer immunotherapy in solid tumors. Cancers (Basel) 2016;8:106. [Crossref] [PubMed]
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974-82. [Crossref] [PubMed]
- Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy 2016;8:821-37. [Crossref] [PubMed]
- Maio M, Grob J, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015;33:1191-6. [Crossref] [PubMed]
- Calabrò L, Danielli R, Sigalotti L, et al. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol 2010;37:460-7. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9. [Crossref] [PubMed]
- Calabrò L, Ceresoli GL, Di Pietro A, et al. CTLA4 blockade in mesothelioma: finally a competing strategy over cytotoxic/target therapy? Cancer Immunol Immunother 2015;64:105-12. [Crossref] [PubMed]
- Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18:1261-73. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 2012;366:2443. [Crossref] [PubMed]
- Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol 2010;7:389-95. [Crossref] [PubMed]
- Balar AV, Weber J. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 2017;66:551-64. [Crossref] [PubMed]
- Awad MM, Jones RE, Liu H, et al. Cytotoxic T cells in PD-L1-positive malignant pleural mesotheliomas are counterbalanced by distinct immunosuppressive factors. Cancer Immunol Res 2016;4:1038-48. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. [Crossref] [PubMed]
- Khanna S, Thomas A, Abate-Daga D, et al. Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti–PD-L1 antibody avelumab. J Thorac Oncol 2016;11:1993-2005. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Kindler HL, Karrison TG, Rose B, et al. Biomarkers of pembrolizumab (P) activity in mesothelioma (MM): Results from a phase II trial. J Clin Oncol 2017;35:abstr 8557.
- Quispel-Janssen J, Zago G, Schouten R, et al. A Phase II study of nivolumab in malignant pleural mesothelioma (NivoMes): with translational research (TR) biopsies. J Thorac Oncol 2017;12:S292-3. [Crossref]
- Goto Y, Okada M, Kijima T, et al. A phase ii study of nivolumab: a multicenter, open-label, single arm study in malignant pleural mesothelioma (MERIT). International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer: Yokohama, Japan. 2017.
- National Institutes of Health. Checkpoint blockade for inhibition of relapsed mesothelioma (CONFIRM): a phase III double-blind, placebo controlled trial to evaluate the efficacy of nivolumab in relapsed mesothelioma. NCT03063450. Available online: http://www.clinicaltrials.gov
- Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016;34:abstr 8503.
- Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015;194:950-9. [Crossref] [PubMed]
- Larkin J, Hodi FS, Wolchok JD. combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:1270-1. [Crossref] [PubMed]
- Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab in combination with durvalumab in first or second-line mesothelioma patients: safety analysis from the phase II NIBIT-MESO-1 study. J Clin Oncol 2017;35:abstr 8558.
- Scherpereel A, Mazieres J, Greillier L, et al. Second- or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: results of the IFCT-1501 MAPS2 randomized phase II trial. J Clin Oncol 2017;35:abstr LBA8507.
- Kelly RJ, Sharon E, Pastan I, et al. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther 2012;11:517-25. [Crossref] [PubMed]
- Hassan R, Thomas A, Alewine C, et al. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol 2016;34:4171-9. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]
- National Institutes of Health. Study of the safety and efficacy of amatuximab in combination with pemetrexed and cisplatin in subjects with unresectable malignant pleural mesothelioma (ARTEMIS). NCT02357147. Available online: http://www.clinicaltrials.gov
- Blumenschein GR, Hassan R, Moore KN, et al. Phase I study of anti-mesothelin antibody-drug conjugate anetumab ravtansine (AR). J Clin Oncol 2016;34:abstr 2509.
- National Institutes of Health. Phase II anetumab ravtansine as second line treatment for malignant pleural mesothelioma. NCT02610140. Available online: http://www.clinicaltrials.gov
- Kindler HL, Novello S, Fennell D, et al. Randomized phase II study of anetumab ravtansine or vinorelbine in patients with metastatico pleural mesothelioma. International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer: Yokohama, Japan. 2017.
- Zhao XY, Subramanyam B, Sarapa N, et al. Antibody therapeutics targeting mesothelin in solid tumors. Clin Cancer Drugs 2016;3:76-86. [Crossref] [PubMed]
- Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus iv infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13:5144-9. [Crossref] [PubMed]
- Kreitman RJ, Hassa R, Fitzgerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res 2009;15:5274-9. [Crossref] [PubMed]
- Hassan R, Miller AC, Sharon E, et al. Major cancer regression in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013;5:208ra147. [Crossref] [PubMed]
- Hollevoet K, Mason-Osann E, et al. In vitro and in vivo activity of the low- immunogenic anti-mesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther 2014;13:2040-9. [Crossref] [PubMed]
- Morello A, Sudelain M, Adusumilli PS, et al. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 2016;6:133-46. [Crossref] [PubMed]
- Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mous model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012;18:2478-89. [Crossref] [PubMed]
- Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD-4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151. [Crossref] [PubMed]


