National adoption of video-assisted thoracoscopic surgery (VATS) lobectomy: the Italian VATS register evaluation
Introduction
The first VATS lobectomy was performed in 1991 (1). Since then, the implementation towards a minimally invasive thoracic surgical strategy has been rather slow. However, the Society of Thoracic Surgery Database showed yet in 2006 a 32% rate of video-assisted thoracoscopic surgery (VATS) lobectomies (2). Reviews of United States National Databases revealed that the percentage of lobectomies done by VATS was less than 6% of the total lobectomies done in 2004 to 2006 and increased to more than 40% in 2010. The data reported from high-volume academic centres showing that more than 90% of their lobectomies are performed through the VATS approach (3-7). Despite this, the percentage was probably not worldwide representative because the database mentioned above included only academic departments and the implementations process was not uniform according to different countries. Contrary to suggestions that VATS is associated with an increased risk of complications compared with open operations, a substantial body of manuscripts accrued (including several large published studies and systematic reviews) promoting the benefits of the VATS lobectomy and comparing the VATS approach with open anatomic lung resection. The current literature suggests VATS may offer superior perioperative outcomes compared with open thoracotomy in propensity-matched patients, with equal long-term oncological and survival outcomes (8,9). Notwithstanding, the slow transition from thoracotomy to video-assisted surgery, despite the obvious advantages, is considered to be due to a demanding learning curve and skills acquisition to face with unexpected intraoperative complications, such as bleeding. Skill acquisition or competence is the benchmark by which physicians are permitted to perform procedures safely and independently via an elaborate operation entail of a sufficient number of processes. For minimally invasive lobectomy, this appears to require a minimum of twenty to thirty cases, with estimates as high as fifty operations (10-12). Determining the significant case numbers to attain procedural skills is important but should not be strictly considered; in other words, newly trained surgeons should be mentored until they demonstrate outcomes that exceed those required for competency. However, progressing to proficiency is a complicated procedure that not only necessitates substantial operative experience but also requires a qualitative leap in knowledge and performance in a proper centre, with an experienced VATS surgeon or supporting staff and with optimal instrumentation. In this setting, different parameters may be used as a reference for a learning curve on VATS lobectomies: the index of complications, the mortality rate, the length of stay, and the rate of conversion to open thoracotomy. The latter seems to significantly reduce once exceeded the threshold above of fifty procedures, although this parameter is cause for debate as it is not closely related to the number of interventions.
Methods
The National Register for VATS lobectomy established in 2013 was used to collect data since January 2014 from 65 Thoracic Surgery Units. Out of more than 3,700 patients enrolled, only information from units with ≥100 VATS lobectomies carried out exclusively by two surgeons were retrospectively analyzed. For statistical reasons, the patients were divided into three chronological groups to compare and evaluate the data regarding the learning curve. The performing surgeons were already experienced in open lobectomies, minor VATS procedures and major VATS excision between 10 and 20 interventions per year. As this was a retrospective review and there was no modification in patients’ care (no prospective randomised study), we did not need the ethic approval in our institutions. Statistical analysis was realised with the bootstrap method using 1,000 pure bootstrap samples with 95% CI. Bootstrap analysis was proposed as a breakthrough method for internal validation of surgical regression models, allowing to approximate the distribution of variables (mean and variance) and to make data derived from the classification of patients based on chronology uniform. Characteristics of the patients were compared using the independent samples Student’s t-test (for age and lung function) and the Bravais-Pearson’s χ2 test or the Fisher’s exact test when appropriate (for gender, and removed lobe). Independent samples t-tests were used to compare the procedures performed in the groups and the ANOVA test (13) was applied from intermediary data. To explore a possible development in conversion rate and complications as the surgeons progressed along the learning curve, they were plotted, and the correlations were calculated using the Spearman’s Rank-Order Correlation. The learning curve technique can be applied to any surgical process with a binary outcome. It is understandable that after a limited number of surgical procedures, both the operation times and complication rates decline; nevertheless, in VATS lobectomies the estimated number of procedures that have to be performed until the learning curve is saturated has not been calculated. The cumulative sum (CUSUM) method (14) was used for the binary performance data control so in this study, the acceptable failure rate for the operation time was set at 50%. The acceptable failure rate for postoperative complications was set at 5%. The unacceptable failure rate for the operation time was set at 70%, for postoperative complications at 10%. This assumption was supported by considering the data in our study and usually acceptable or unacceptable standards. R version 3.2.3 was used for statistical analysis (R Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/).
Results
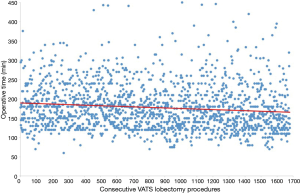
Ten institutions contributed a total of 1,679 patients (Table 1). Three groups of patients were established according to the sequence of surgery (602 in 2014, 614 in 2015, 463 in 2016, Table 2). There were no differences between groups regarding age, the Charlson index, the length of utility thoracotomy or intraoperative blood loss. The length of postoperative stay was 4±1 days. The mean operative time was reduced during the observed period (Figure 1), albeit the differences were not statistically significant. The conversion rate to thoracotomy (overall 7.44%) decrease during the learning curve 9.36% in the Group 1, 6.84% in Group 2, 5.39% in Group 3 showing a significant learning reduction (P=0.048). The reasons for conversion to thoracotomy were vascular injuries (17 patients of 58 in Group 1, 11 patients of 42 in Group 2, 4 patients of 25 in Group 3), unfavourable anatomy and cancer invasion of the pulmonary artery. The most common complication consisted in prolonged air leak; nevertheless, interpolation line showed a significant reduction due to expertise (P=0.00086). The need of postoperative transfusions decreased between Group 1 and Group 3 (P=0.023) while the rate of haemothorax remained constant during the learning curve. Complications were classified at Grade II according to Clavien-Dindo Classification (15) except for vascular lesions classified at Grade IIIb. CUSUM of the quality characteristic index (Figure 2) certified the validity of the frequency and probability distribution of variables data as the deviations from the mean value were minimal.
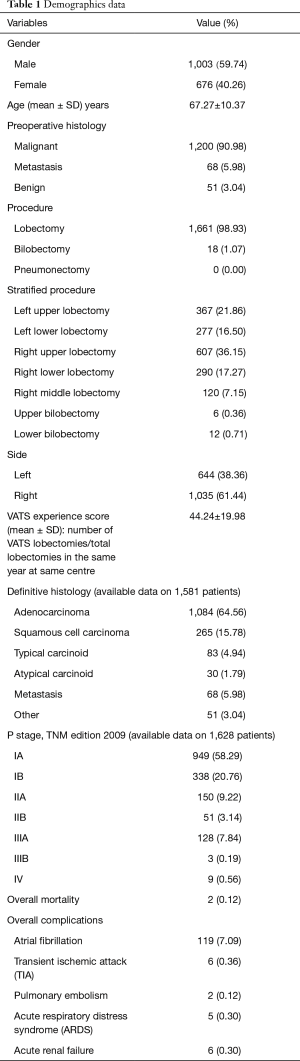
Full table
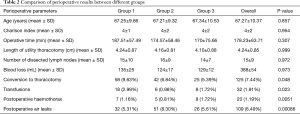
Full table
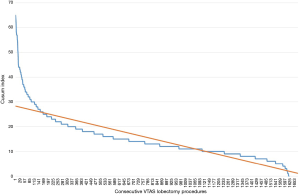
Discussion
Since the introduction of VATS lobectomy (1,16,17), it has become a standard procedure worldwide for lung cancer treatment. Although minimally invasive surgery improves patient recovery with shorter length without compromising surgical outcomes, like oncological prognosis or surgical morbidity (8,9), it is still underused due to perceived technical challenges compared to an open approach and learning curve. Moreover, 2% to 9% of VATS lobectomies are converted to open thoracotomy (18,19). But, if the causes of conversion are described such as intraoperative complications, bleeding, technical or anatomical problems, conversion-related factors and the impact of learning curve have been poorly investigated, as well as the effect of caseloads during skill acquisition time (20). Petersen et al. (21) studied conversion and its reduction in VATS lobectomy during their early and late experience. Authors conclude that an increased experience, a safer learning curve under guided supervision by an experienced VATS surgeon with highly selected training cases and improved technologies (e.g., high definition cameras, three-dimensional technology, VATS-tailored curved instruments, staplers), resulting in a dramatic decrease in complication rates. However, VATS lobectomy is a technically challenging operation that requires extensive training for optimal performance. Proper mentoring by experienced surgeons in the technique, along with adequate surgical volume, substantially affect the length of the learning curve needed to master this procedure. Although the suggested duration of the learning curve is of fifty video-assisted lobectomies (22), several factors influence skill acquisition. The first of all is the presence of an experienced VATS surgeon as a visual supervisor and then the centre volume, which can ensure a sufficient number of procedures in a short time frame. Also, a high-volume centre allows the loading of many potential selected cases for training. In this setting, since most lobectomies are performed by VATS, an ongoing discussion is about how future trainee surgeons are going to learn to do VATS lobectomies. In fact, if in the past there was a gradual transition from thoracotomy to minimally invasive procedures, nowadays the open approach is scheduled preferentially for complicated procedures with associated surgical manoeuvres, such as partial chest wall resection or post-adjuvant resections with higher recurrence of adhesions, thus leading to a “technical debt” for inexperienced or trainee surgeons in dissection, release, isolation and section manoeuvres of the hilar structures. For these reasons, open surgery to inexperienced surgeons should be a priority and demand without considering their propensity or talent to learn more quickly than others. At first, the introduction of VATS lobectomies was performed by self-taught surgeons experienced in open surgery, who in the case of intraoperative difficulties, were immediately able to shift to thoracotomy, with a high conversion rate (23). But, this model seems to have been exceeded, as reported by Konge et al. (11), by a supervised first-stage approach to trainees without any experience in open surgery. However, training in traditional thoracic surgery is the essential precondition for later training in VATS and makes surgeons ready for emergency conversion to open thoracotomy, in the case of unexpected bleeding or structure avulsion. Moreover, the transition from open to minimally invasive surgery should be considered a milestone aspect of the VATS learning program. Ra et al. (12), examining an experienced single surgeon who performed 38 lobectomies (14 VATS, 14 open thoracotomy and 10 VATS conversions to open), reported an increased number of VATS lobectomies from the second to the third quarters of the study with a decreased number of conversions. Authors concluded changes reflected a statistically significant progression of the surgeon on his learning curve (P=0.002). The impact of the learning curve of VATS lobectomy conversion has been widely defined. As known, the number of patients needed to learn a surgical skill is variable, though fifty VATS lobectomies and hundred VATS procedures are recommended (22). As previously mentioned, centre volume has to be considered as the principal limit. In other words, a VATS lobectomy program should be embarked upon only in the potentiality to perform at least 25 VATS lobectomies for each surgeon. Moreover, the introduction of VATS lobectomy in a clinical setting should be restricted to few surgeons to complete learning curves for the first generation of VATS surgeons in a reasonable time (23). However, the number of procedures should be considered both for efficiency, and consistency. Li et al. (24) studying 200 patients who underwent VATS lobectomy evaluated learning curves for operative time, blood loss, and postoperative length of stay, concluding that between 100 and 200 cases are required to achieve efficiency in these variables and consistency need even more cases. Regarding VATS conversion, they presented higher rates of conversion to open surgery in the first cases of the study. Considering these aspects, we proposed a statistical analysis dividing our whole series into three periods, according to the possible impact of time and case load and so the achieved surgeon’s expertise and confidence, to understand the real implications of the learning curve in rates of conversion. In the first group which included 602 patients, there was an overall conversion rate of 9.36%. Compared with the other groups (6.84% in Group 2 and 5.39% in Group 3), there was a statistical difference among the periods, reflecting an increased consistency with the minimally invasive technique. According to other experiences, vascular injuries, unfavourable anatomy (e.g., segmental left pulmonary artery dissection for left upper lobectomy) and cancer invasion were the most causes of conversion to open lobectomy (6). However, the amount of intraoperative blood loss did not change during the periods with a light decrease between Group 1 and Group 2 (135 vs. 124 mL), while re-increasing between Group 2 and Group 3 (124 vs. 129 mL). This could be explained by the surgeon’s skill, ability and self-confidence which permit more complex resections. Regarding postoperative morbidity, skill acquisition also reflected on patients’ morbidity and mortality. In particular postoperative air-leakages significantly decreased among the periods (P=0.00086), proving that a careful dissection of the hilar vessels and pulmonary parenchyma, as well as the correct use of staplers both, represent aspects of maturation in the technique. Instead, the decrease in occurrence of postoperative anemia (P=0.023) and the stability of the hemothorax (P=0.0051) during skill acquisition should be attributable to the greater propensity to an extended lymphadenectomy by an experienced VATS surgeon when compared to inexperienced ones referring regularly to lymph node samplings, as the dissection of mediastinal stations (paratracheal, aortopulmonary window and subcarinal) can hide threatening intraoperative sources of bleeding thus leading to an early conversion. Moreover, lymphadenectomy is a technical challenge in VATS. Boffa et al. (25), in a comparative study between open and VATS lobectomy including 11,531 (7,137 open and 4,394 VATS) clinical stage I primary lung cancer patients, showed that the minimally invasive group has a lower rate of N1 upstaging (Open vs. VATS: 9.3% vs. 6.7%; P<0.001), suggesting that the VATS may compromise hilar lymph node clearance. On the other hand, Lee et al. (7), reporting their experience about the effect of the learning curve in hilar-mediastinal VATS lymphadenectomy in 500 patients, showed significantly more lymph nodes were removed in the late group than in the early one, and more N1 and N2 stations were sampled. The new group also had significantly more pathologic N1 and N2 disease (19% vs. 10%, P=0.006). Similarly, Gonzalez et al. (26), analysing their initial 3 years of experience with 200 VATS lobectomy cases by dividing them into three groups according to the year of operation, reported improved lymph node assessment with increased experience. For their first, second, and third groups, the mean number of lymph nodes evaluated was 9.3 vs. 6.1, 10.1 vs. 4.9, and 13.94 vs. 7.3 (P=0.003), and the average number of lymph node stations sampled was 3.65 vs. 1.2, 3.85 vs. 1.2, and 4.57 vs. 1.22 (P<0.001), respectively. In another study, Zhao et al. (10) reported their institution’s initial experience with 90 VATS lobectomies divided into three consecutive groups of 30 patients each. They found no difference in the mean numbers of lymph nodes resected or in the average numbers of nodal stations sampled. This lack of differences in that study may have been due to the small sample size of observed cases. Finally, concerning with changes in operative time, no difference were described among the groups (Group 1: 187.51 min, Group 2: 174.57 min, Group 3: 170.0 min; P=0.307) in our experience. Billè et al. (27) studied the learning curve of VATS lobectomy comparing results between 66 patients (Group A) treated by a thoracic surgeon consultant and 34 patients (Group B) treated by trainees. Thirty-nine patients (59%) in Group A and 22 patients (64.7%) in Group B revealed complete fissure. Authors did not find any difference in: (I) conversion rate (Group A: 6 patients, 9.1% vs. Group B: 3 patients, 8.8%; P=0.6); (II) complication rate (Group A: 24 patients, 36.3% vs. Group B: 11 patients, 32.3%; P=0.4); (III) blood loss (Group A: 200±50 mL vs. Group B: 250±60 mL; P=0.2); (IV) removal of pleural drain [Group A: 3 days, range, (1-26) days vs. Group B: 3 days, range, (1-25) days; P=0.3]; (V) length of hospitalization [Group A: 5.5 days, range, (2–96) days vs. Group B: 5 days, range, (3-20) days; P=0.5]; (VI) procedure timing (Group A: 125±30 min vs. Group B: 133±26 min; P=0.18). Authors concluded that the training program of VATS lobectomy does not worsen outcomes. Referring to our results, surgeons involved were all minimally invasive experienced ones with more than the twenty-five procedures required. In contrast with our results, Zhao et al. (10) reported their investigation on the VATS learning curve of a single surgeon. Patients were divided into three chronological groups of thirty. Authors showed statistical differences in operative time (Group A: 214.2 min, Group B: 153.8, Group C: 148.3 min; P<0.001, respectively) and blood loss (Group A: 285 mL, Group B: 150 mL, Group C: 138.3 mL; P<0.001, respectively) with no differences in conversion rate (P=0.781). This experience, however, refers only to one surgeon; therefore, it could present a selection bias, which is not the case in our sample as it relates to a large number of multi-center surgeons.
Conclusions
In conclusion, the learning curve for VATS lobectomy is a fundamental step for an up-to-date thoracic surgeon, who is called upon to face and know how to manage technological innovations. Skill acquisition cannot be separated from a thorough knowledge of the technique or supervised tutoring. At the same time, despite the recommendations by authoritative groups from Northern Europe, a VATS surgeon must have an established background of the open technique and knowledge of how to deal with dangerous emergency complications, such as breakthrough bleeding. If on the one hand, in a VATS program one is required to be able to remedy complications without resorting to conversion, on the contrary, every contemporary surgeon should consider the limits both of the technique and of himself. Thus, the volume of patients is fundamental to proceed to open and VATS training contemporarily. Therefore, it would be desirable that contemporary VATS surgeons have an ethical and professional responsibility to undertake specialised training in recognised VATS lobectomy institutions and be proctored by experienced surgeons before initiating a VATS program. The learning curve is not an end of training but a beginning of where you need a continuous updating, through specific workshops and simulation programs. In our study, we only considered experienced surgeons in order to reduce the number of outliers in agreement with Rocco considerations (28). This aspect could be a limit to the correct evaluation of the outcomes of our VATS lobectomy training program and could explain why some technical aspects (such as surgical timing) are not to be retained significant.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Lee PC, Kamel M, Nasar A, et al. Lobectomy for Non-Small Cell Lung Cancer by Video-Assisted Thoracic Surgery: Effects of Cumulative Institutional Experience on Adequacy of Lymphadenectomy. Ann Thorac Surg 2016;101:1116-22. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [PubMed]
- Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [Crossref] [PubMed]
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedures is needed to learn lobectomy by video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Ra YJ, Ahn HY, Kim MS. Learning Curve of a Young Surgeon's Video-assisted Thoracic Surgery Lobectomy during His First Year Experience in Newly Established Institution. Korean J Thorac Cardiovasc Surg 2012;45:166-70. [Crossref] [PubMed]
- Nyaga VN, Aerts M, Arbyn M. ANOVA model for network meta-analysis of diagnostic test accuracy data. Stat Methods Med Res 2016. [Epub ahead of print]. [PubMed]
- Noyez L. Control charts, Cusum techniques and funnel plots. A review of methods for monitoring performance in healthcare. Interact Cardiovasc Thorac Surg 2009;9:494-9. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Giudicelli R, Thomas P, Lonjon T, et al. Major pulmonary resection by video assisted mini-thoracotomy. Initial experience in 35 patients. Eur J Cardiothorac Surg 1994;8:254-8. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52; discussion 1252-3. [Crossref] [PubMed]
- Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Gazala S, Hunt I, Valji A, et al. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:962-4. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- McKenna RJ Jr. Complications and learning curves for video-assisted thoracic surgery lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [Crossref] [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Billè A, Okiror L, Karenovics W, et al. Thoracoscopic lobectomy: is a training program feasible with low postoperative morbidity? Gen Thorac Cardiovasc Surg 2013;61:409-13. [Crossref] [PubMed]
- Rocco G. The impact of outliers on the mean in the evolution of video-assisted thoracoscopic lobectomy. Eur J Cardiothorac Surg 2017;51:613-5. [Crossref] [PubMed]


