The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions
Introduction
With the development of computed tomography (CT) and its widespread use for diagnosis, the number of small lesions discovered in the peripheral lung field has increased in recent years (1). It is considered that tissue diagnosis of a peripheral pulmonary lesion (PPL) be conducted before the start of treatment. Diagnostic sampling of a PPL includes transbronchial biopsy (TBB) under X-ray fluoroscopy and percutaneous transthoracic needle aspiration (TTNA). It was reported in the guidelines of the American College of Chest Physician (ACCP) that the diagnostic yield of TBB was 63% for lesions ≥20 mm (n=984), and 34% for lesions <20 mm (n=383) (2). On the other hand, the diagnostic yield of TTNA for a PPL was ≥90%, making it the preferred method for pre-operative diagnosis (2). However, TTNA has been reported to cause complications such as pneumothorax, air embolism, and tumor seeding (3,4). Therefore, it is prudent to search for a more safe diagnostic method.
According to a recent meta-analysis, the diagnostic yield of guided bronchoscopy using new techniques such as virtual bronchoscopic navigation, endobronchial ultrasonography with a guide sheath (EBUS-GS), and ultrathin fiber was ≥70%. The most common complication was pneumothorax but even so, the rate of it occurring was low at 1.5%, indicating that TBB is a safe diagnostic method (5). However, these reports were based on data involving solid nodules. Recently, with the widespread use of CT, interest on ground glass opacity pulmonary lesions (GGO) has been increasing (1). GGO comes in two kinds: pure GGO and mixed GGO, the former has no solid component while the latter has a solid component inside. The frequency of discovering GGO has been increasing and the diagnostic method has posed a clinical problem in recent years (6,7).
Chest tomosynthesis (the SONIALVISON safire radiography/fluoroscopy system, Shimadzu, Japan) is a term coined from “tomography” and “synthesis”. It is a device that permits reconstruction of several slices of the thorax in the coronal plane at a desired depth in a single session of photography. Currently, it is used mainly in the field of orthopedics but there has been a recent report that it is excellent in visualizing chest nodules (8). Likewise, Terzi et al. reported in the SOS study that chest tomosynthesis was very useful to detect early lung cancer (9). In addition, it was recently found to be valuable for identifying the site of a GGO when performing EBUS-GS (10).
To our knowledge, there have been some reports on surgery or TTNA for the diagnosis of GGO, but none on TBB (11,12). Thus, the purpose of this study was to evaluate the diagnostic yield of EBUS-GS and the utility of tomosynthesis for PPLs with GGO.
Materials and methods
Patient enrollment
We retrospectively reviewed the medical and imaging records of all patients who underwent guided bronchoscopy for PPLs at our institution between July 1, 2012, and October 31, 2012. All diagnostic procedures were performed upon the request of pulmonary physicians or surgeons. The diagnostic method (i.e., TBB, CT-guided TTNA, or surgical biopsy) was determined on an individual basis depending on the radiologic and clinical features and the views of the patient. All patients had 5 mm-slice chest CT scan done within four weeks of the procedure and additional 1-mm thin section chest CT scan using an 80-detector CT (Aquillion PRIME, TOSHIBA, Tokyo, Japan). Images were displayed with a lung window setting (center, –600 H; width, 1,500 H).
There was a total of 364 patients in the study period, but only those who had chest CT scan findings of GGO, defined as an area of increased attenuation without obscuring the underlying vessels and bronchi (13) were included. Data collected were diameter of the lesion (<20 vs. ≥20 mm) and percentage of the GGO component (<50%, ≥50%, pure GGO). A pure GGO was defined as a lesion with no solid part while a GGO-dominant lesion was defined as a lesion with a GGO ratio of more than 50%.
This study was approved by the National Cancer Center Institutional Review Board (No. 2012-199).
Procedure of EBUS-GS for GGO
The location of the bronchi leading to the lesion was planned by reviewing chest HRCT images prior to bronchoscopy. In addition, we prepared coronal plane tomosynthesis images before the start of every bronchoscopy procedure and took note of the location of the lesion in relation to the other structures of the thorax. This tomosynthesis image was placed side by side with the fluoroscopy screen to serve as a guide during the EBUS-GS procedure.
For all patients, flexible bronchoscopy was done using a fiberoptic bronchoscope (BF-1T260 or BF-P260F, Olympus, Japan) in combination with a radial ultrasound probe (UM-S20-20R or UM-S20-17S, Olympus, Japan) and a guide sheath kit (K-201 or K-203, Olympus, Japan). The procedure was done under local anesthesia with conscious sedation and the scope was inserted through the oral route, in the same way as the usual bronchoscopy.
Upon reaching the target bronchus, the guide sheath together with an ultrasound probe was inserted through the working-channel of the scope and advanced, under fluoroscopy guidance (VersiFlex VISTA, Hitachi, Japan), towards the area indicated by the previously prepared tomosynthesis image.
We described the EBUS findings according to Kurimoto et al., who classified EBUS signals into three groups based on internal echoes generated by the pathologic tissue and its surrounding structures (14). These groups are further subdivided according to the morphology of the hyperechoic areas, and the presence or absence fluid-filled vascular spaces or air in the patent bronchioles and alveoli. The classes of lesions are as follows: type I, homogeneous pattern (type Ia, with patent vessels and patent bronchioles; type Ib, without vessels and bronchioles); type II, hyperechoic dots and linear arcs pattern (type IIa, without vessels; type IIb, with patent vessels); and type III, heterogeneous pattern (type IIIa, with hyperechoic dots and short lines; type IIIb, without hyperechoic dots and short lines).
If the target location was not detected by EBUS, the EBUS probe was manipulated under fluoroscopy guidance until an acoustic signal was generated. While doing so, we made sure to stay in the lung segment corresponding to the previously mapped tomosynthesis image.
After confirming the location of the guide sheath by EBUS, repeated diagnostic sampling was done by TBB, bronchial brushing, and flushing of the guide sheath with 2 mL of saline (EBUS-GS-guided sampling). Fluid samples were sent for cytology and TBB samples were sent for histopathologic examination. For the EBUS-GS-guided samples that were diagnosed as inflammatory change on pathological examination, follow-up CT imaging was done after three months; those lesions that resolved were designated as benign. If a definitive diagnosis was not established, the patient underwent surgical biopsy to confirm the diagnosis of the GGO lesion.
Statistical analysis
Descriptive statistics were presented as frequency, percentage, and mean ± standard deviation (SD). We investigated the factors affecting diagnostic yield using Fisher Exact Test. Variables with P-values less than 0.2 were analyzed using logistic regression. All P-values were two sided and levels ≤0.05 were considered statistically significant. Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html; Kanda), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, Ver. 2. 13.0) and a modified version of R commander (Ver. 1.8-4).
Results
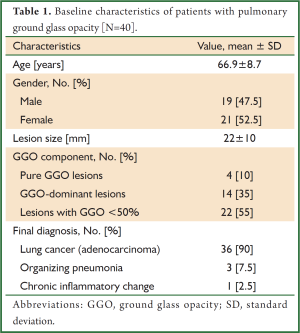
The study population included 40 patients (19 men, 21 women; age 66.9±8.7 years, mean ± SD) who had peripheral pulmonary lesions with GGO; 35 were detected during lung cancer screening and five patients were detected during studies for clinical reasons. The mean lesion diameter was 22±10 mm (mean ± SD) (Table 1). There were 24 lesions characterized as either type IIa (n=23) or type IIIa (n=1) (14). Sixteen cases did not fit the characteristics described by Kurimoto’s classification.
Full table
Final diagnosis in the majority of cases was adenocarcinoma; a few cases were organizing pneumonia and chronic inflammatory change (Table 1). EBUS-GS-guided TBB was diagnostic in 26 cases (65.0%), the results of which were as follows: primary lung cancer/adenocarcinoma (n=25), and chronic inflammatory change (n=1). The cytological specimen from EBUS-GS-guided bronchial brushing and flushing of the guide sheath was diagnostic in only 14 cases (35.0%). Lesions that were diagnosable by cytology were also diagnosable by TBB. Mean number of specimens obtained using EBUS-GS-guided TBB was 6.1±2.2 (mean ± SD). Average duration of procedure was 26.3±7.3 minutes (mean ± SD). EBUS-GS-guided TBB was non-diagnostic in 14 cases; these were subsequently diagnosed as primary lung adenocarcinoma (n=11) and organizing pneumonia (n=3) by surgical resection.
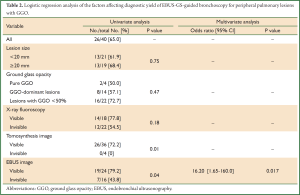
The overall diagnostic yield of EBUS-GS-guided TBB was 65.0% (26 of 40 lesions). By univariate analysis (Table 2), diagnostic yield did not vary significantly with size of the lesion (P=0.75), percentage of the GGO component (P=0.47), detectability on fluoroscopy (P=0.18), and location of the EBUS probe in relation to the lesion (P=1.0), but was significantly higher when the target lesion was detectable by tomosynthesis (P=0.01), and when an EBUS image was generated during scanning of the target site (P=0.04).
Full table
By multivariate analysis (Table 2), the presence of an EBUS image [odds ratio 16.2 (1.65-160.0), P=0.017] was the only factor that significantly affected the diagnostic yield.
For all 40 patients, EBUS-GS-guided TBB had only one complication: pneumothorax that did not require chest tube draining. Major bleeding, pneumonia, or air embolism was not detected.
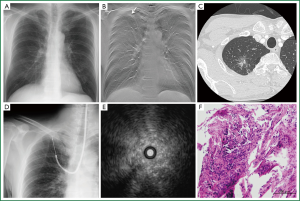
A representative case is shown in Figure 1. A 59-year-old man with a chest CT showing a mixed GGO measuring 24 mm in the major axis in the right S1a was admitted to our hospital. Note that the GGO was not seen on plain radiography (Figure 1A). Prior to bronchoscopy, tomosynthesis images were prepared to map the location of the target site in the coronal plane (Figure 1B). The endobronchial route was planned by reviewing HRCT images (Figure 1C). The EBUS probe was advanced into the B1ai bronchus and manipulated under fluoroscopy guidance (Figure 1D) towards the area indicated by the previously prepared tomosynthesis image. EBUS scanning demonstrated a pattern of low-echoic lesion with hyperechoic dots and linear arcs without vessels (Figure 1E). Histopathological specimen from the TBB showed cuboidal tumor cells lining the entrapped alveolar space.
Discussion
To our knowledge, this is the first report on the diagnostic utility of EBUS-GS and chest tomosynthesis images for GGO, which are now being detected more frequently with the advent of CT screening. Most GGOs are from an inflammatory change but some that turn out to be malignant are usually adenocarcinoma by histology (13). Thus, it is important to distinguish when is a GGO adenocarcinoma or not.
So far, for solid peripheral pulmonary lesions, bronchoscopy or TTNA are the usual procedures for tissue diagnosis but the primary choice is the latter because of a diagnostic accuracy of ≥90% (5). It was reported recently that a similar diagnostic accuracy of TTNA holds true for GGO, regardless of the size, (>20 vs. <20 mm) (13,15). However, TTNA poses a 15-35% risk of pneumothorax and other complications such as, air embolism and tumor seeding (3,4). According to a study on Japanese bronchoscopy, TBB for peripheral lung lesions has a complication rate of only 1.55% (937/60,257 cases). The complications reported were pneumothorax (0.44%) and respiratory failure (0.04%). These suggest that the risks associated with TBB are very low as compared with TTNA (16).
For solid peripheral pulmonary lesions, guided bronchoscopy using EBUS and a guide sheath was developed and consequently improved the diagnostic yield of TBB as compared to traditional TBB under fluoroscopy alone (5). This can be attributed to the capability of this imaging modality to characterize a solid nodule or a consolidation (17-21). By far, there has been no report on the diagnostic yield of TBB for GGO. This is because of the difficulty of confirming the location of a GGO lesion using fluoroscopy alone.
In our study, the diagnostic yield of EBUS-GS-guided TBB for GGO was 65%. This is not different from the diagnostic yield of TBB for solid nodules described in the ACCP Guidelines and almost similar to the one reported by a meta-analysis on guided bronchoscopy (2,5). In addition, the diagnostic yield was 68.4% for GGO ≥20 mm and 61.9% for GGO <20 mm, which did not reach a statistically significant difference. This is similar to the previous reports on TTNA for GGO, which means that the diagnostic yield of EBUS-GS-guided TBB for GGO is not affected by the size of a lesion (13).
Confirmation of the location of a lesion by TTNA is easy because it involves a more detailed radiologic imaging. However, when using the bronchial access to a peripheral tumor, confirmation of the location of lesion is conventionally by X-ray fluoroscopy. Whether or not a lesion can be visualized on X-ray fluoroscopy was cited to be one of the very important factors that affect diagnosis by TBB (2). But our results suggest otherwise; diagnostic yield of EBUS-GS-guided TBB for GGO lesions was not affected by detectability on fluoroscopy. What is important in the diagnosis of GGO then? Our data showed that accurate confirmation of the location of a lesion by EBUS is of utmost importance for the diagnosis of GGO using EBUS-GS-guided TBB, with a yield of 79.2% compared to a 43.8% diagnostic yield when EBUS image was not detected (P=0.017).
It has been reported that tomosynthesis is significantly better than radiography at visualizing small nodules in the peripheral lung (8,22). In this study, tomosynthesis was conducted in advance and used for mapping the location of the target lesion in the coronal plane. Real time tomosynthesis cannot be used for guided bronchoscopy at present because of the time necessary for image reconstruction. Consequently, the benefits of tomosynthesis are not maximized yet. For the improvement of the diagnostic accuracy of TBB for GGO, it is useful to develop real-time tomosynthesis that can be used while conducting guided bronchoscopy (10).
Regarding EBUS, Kurimoto’s classification of solid nodules was reported and is considered useful for detecting a lesion (14). However, there has not been an established classification of EBUS images for GGO. We observed that type IIa in Kurimoto’s classification accounted for 57.5% and type IIIa accounted for 2.5%. Furthermore, there were many cases in which EBUS had much difficulty in visualizing GGO, especially pure GGO and GGO-dominant lesions. The reason for this is that, EBUS generates an acoustic image from the area the probe contacts. Thus, the EBUS image depends on which part of the lesion (solid or GGO) the probe is in. Solid part presents as either type II or type III. Another reason is that the pathologic change of a pure GGO or a GGO-dominant lesion is either too small or is in the beginning stage thus, cannot be perceived by ultrasound. In any case, further study on EBUS images of GGO is required.
In this study there are several limitations. First, it is a retrospective analysis in a single institute and it is not randomized. Second, the bronchoscopy procedures were not performed by the same bronchoscopist. Prospective, randomized trials on TBB for GGO that take into account these confounding variables are needed in the future.
Conclusions
TBB through EBUS-GS can be considered as one of the diagnostic methods for GGO. The diagnostic yield is high particularly when the location of a lesion can be confirmed by EBUS. Further technological improvements are required to identify the location of the target GGO lesion more precisely and consequentially enhance diagnostic yield.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Detterbeck FC, Homer RJ. Approach to the ground-glass nodule. Clin Chest Med 2011;32:799-810. [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [PubMed]
- Goo JM, Park CM, Lee HJ. Ground-glass nodules on chest CT as imaging biomarkers in the management of lung adenocarcinoma. AJR Am J Roentgenol 2011;196:533-43. [PubMed]
- Vikgren J, Zachrisson S, Svalkvist A, et al. Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: human observer study of clinical cases. Radiology 2008;249:1034-41. [PubMed]
- Terzi A, Bertolaccini L, Viti A, et al. Lung cancer detection with digital chest tomosynthesis: baseline results from the observational study SOS. J Thorac Oncol 2013;8:685-92. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The value of chest tomosynthesis in locating a ground glass nodule (GGN) during endobronchial ultrasonography with a guide sheath: a case report. J Thorac Dis 2013;5:E75-7. [PubMed]
- García-Río F, Pino JM, Terreros-Caro JG, et al. Pneumothorax risk prediction in lung needle biopsy by pulmonary function tests. Chest 1997;111:1141-2. [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [PubMed]
- Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest 2002;122:1887-94. [PubMed]
- Lu CH, Hsiao CH, Chang YC, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143-50. [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Bronchoscopic practice in Japan: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2013;18:284-90. [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [PubMed]
- Fielding DI, Robinson PJ, Kurimoto N. Biopsy site selection for endobronchial ultrasound guide-sheath transbronchial biopsy of peripheral lung lesions. Intern Med J 2008;38:77-84. [PubMed]
- Shinagawa N, Yamada N, Asahina H, et al. Transbronchial Biopsy for Peripheral Pulmonary Lesions Under Real-time Endobronchial Ultrasonographic Guidance. J Bronchology Interv Pulmonol 2009;16:261-5. [PubMed]
- Fielding DI, Chia C, Nguyen P, et al. Prospective randomised trial of endobronchial ultrasound-guide sheath versus computed tomography-guided percutaneous core biopsies for peripheral lung lesions. Intern Med J 2012;42:894-900. [PubMed]
- Shinagawa N, Nakano K, Asahina H, et al. Endobronchial ultrasonography with a guide sheath in the diagnosis of benign peripheral diseases. Ann Thorac Surg 2012;93:951-7. [PubMed]
- Yamada Y, Jinzaki M, Hasegawa I, et al. Fast scanning tomosynthesis for the detection of pulmonary nodules: diagnostic performance compared with chest radiography, using multidetector-row computed tomography as the reference. Invest Radiol 2011;46:471-7. [PubMed]


