Engineered circulatory scaffolds for building cardiac tissue
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality, producing immense health and economic burdens in United States and globally (1,2). The adult cardiomyocytes (CMs), terminally differentiated, have extremely limited regeneration capacity, which restrains the potential to restore cardiac function after myocardial infarction (MI), eventually leaving millions of people worldwide vulnerable to heart failure (HF) (3,4). HF is the terminal state of CVD, and the optimal choice used clinically is heart transplantation. However, the paucity of appropriate donors severely constrains the number of patients receiving transplantation, resulting in unnecessary deaths and sufferings (5). Therefore, cardiac tissue engineering, a new approach to facilitate cardiac repair, after MI, is urgently needed.
The terminal goal for cardiac tissue engineering is growing a whole functional heart to compensate the shortage of heart sources in the lab and the field is currently subdivided to three sections: cardiac patches, vasculature, and valves (6,7). A large number of researches on building cardiac patches in vitro have been done, but the key challenge is the limited thickness of cardiac patches, which results in the implants failed to engraft and survive due to the incompetence of inosculation with host vasculature. Thus, the combination of cardiac and vascular tissue engineering would be efficient and attractive.
Cardiac tissue has evolved a complex internal microvasculature, consisted of endothelial cells (ECs), fibroblasts and pericytes (8), which provides nutrients and oxygen and removes wastes for CMs to survive and function normally (9,10). Thus, in order to achieve the therapeutic potential of the bioengineered cardiac patch, vascularization of the patch is a prerequisite for maintaining its viability after engraftment (10,11). Circulatory scaffold is one of approaches to engineer vascularized cardiac tissue in vitro and expected to integrate with host cardiac tissue to promote its pumping function in vivo. Furthermore, heart-on-a-chip, based on the circulatory scaffolds, is attracted as a versatile bioreactor to further recapitulate cardiac hierarchical architecture and its native microenvironments to broaden the research for building cardiac tissue. Herein, we review different strategies to engineer circulatory scaffolds for building cardiac tissue with microvasculature and assemble such perfusable scaffolds into heart-on-a-chip system, and discuss its current state and future direction.
Basic knowledge, evidence and necessity for vascularization
The developmental biology: vasculogenesis and angiogenesis in vivo
Vasculogenesis, emergence of blood vessels de novo in embryogenesis, begins with differentiation of mesodermal progenitors into angioblasts (12). With the stimulation of angiogenic factors like vascular endothelial growth factor (VEGF), angioblasts subsequently differentiate into ECs, which assemble into primary vascular plexus consisting of fine capillaries (12). Subsequently, the plexus is physiologically remolded and matured into the circulatory system via the process of angiogenesis (13). Differently, angiogenesis, vascular sprouting, is the process of constructing newly blood vessels which currently is known to be included by three steps-vessel branching, maturation and quiescence-with the maintenance signal, such as VEGF, NOTCH, and angiopoietin-1 (ANG-1) (14). To date, vasculogenesis have been elucidated to not be restricted to early embryogenesis with the discovery of circulating ECs and endothelial progenitor cells (EPCs), which probably co-occur with angiogenesis not only in the development, but also in several pathological conditions, such as tumors or vascular diseases (15), especially in MI (16).
Left cardiac ventricle will experiences a series of tissue remodeling after acute MI, which can be divided into two phases, inflammatory phase and reparative phase (17). Specifically, necrotic CMs and matrix debris are cleared by macrophages and neutrophils during the inflammatory phase, and the reparative phase is consisted of inflammation resolution, neovascularization and scar formation. More importantly, angiogenesis is probably the main mechanism of neovascularization during the formation of the granulation tissue observed mainly on the border zone, which is not enough to generate broad microvessels to meet totally healing demand (18,19). However, recent studies have proven that vasculogenesis and angiogenesis could be induced in vitro to construct microvasculature (20,21), which can be targeted to combine with cardiac tissue engineering to increase the amount of microvessels to meet demands of dynamic heart for amelioration of impaired myocardium.
The limitation of scale-up cardiac tissue in vitro
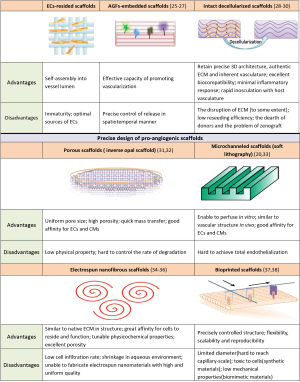
Despite the advances in engineering heart tissues (EHTs) in vitro, the thickness of cardiac tissue, all the time, has been impeding the clinical translation of cardiac tissue engineering. To our best knowledge, the authentic thickness of cardiac construct in vitro, which wound have a physiologic cell density, was confined to about 200 µm due to the limited oxygen diffusion (22). In native cardiac tissue, the functional intercapillary distances are at an average distance of 20 µm such that each myofiber is compactly parallel with and directly supplied by two capillaries (23). Obviously, the density of microvessels in vitro cardiac construct is far lower than that in vivo; thereby scale-up of cardiac tissue for clinical application is still challenging and elusive. In order to transcend the limitation, the notion of novel pro-angiogenic scaffolds (24), cooperated with the mechanisms of vasculogenesis and angiogenesis, has been conceived to engineer circulatory scaffolds to build thicker cardiac tissue discussed as follows. Figure 1 shows comparison of several circulatory scaffolds for engineered cardiac tissue.
Strategies of circulatory scaffolds for engineered cardiac tissue
ECs-resided scaffolds
ECs account for the main proportion (60%) of non-myocytes in the heart (8), which are regarded as a significant cell source for constructing microvasculature in vitro to perfuse the scaffolds seeded with CMs to maintain viable cardiac patch. Over the past years, a large number of efforts has been made to build circulatory scaffolds integrated with microvessels by the addition of ECs alone or with other supporting cells (10,20,39-41). Originally, Narmoneva et al. (41) firstly reported the co-culture of CMs and ECs on the peptide hydrogel scaffold, demonstrating that the addition of ECs could construct capillary-like network and improve neonatal CM survival, spatial organization and synchronized contraction. These studies imply that ECs play an essential and significant role in the building of perfusable cardiac tissue, whereas it is still fraught with challenges.
The source of ECs
The source of ECs could be categorized into two groups, primary ECs and EPCs induced by pluripotent stem cells (PSCs). Primary ECs mainly consist of human umbilical vein ECs (HUVECs) and human microvascular ECs (HMVECs), enabling self-assembly to form vascular networks encompassed with CMs. HUVECs are the most usually utilized in the building of circulatory scaffolds (42,43). Lesman et al. constructed tissue-engineered human vascularized cardiac muscle ex vivo using the triculture of human embryonic stem cell (hESC)-derived CMs, HUVECs and embryonic fibroblasts (EmFs) onto biodegradable porous scaffolds (42), which then were transplanted to the in vivo rat heart and confirmed the functional integration between in vitro blood vessels and host coronary vasculature, forming stable grafts. Moreover, Valarmathi et al. engineered a 3D prevascularized collagen cell carrier (CCC) scaffold by the combination of human cardiac microvascular endothelial cells (hCMVECs) and human mesenchymal stem cells (hMSCs) for co-culture of the human induced pluripotent stem cell-derived embryonic cardiac myocytes (hiPSC-ECMs) and the human adipose-derived multipotent mesenchymal stem cells (MSCs) (44). Regardless of the advanced results in primary ECs, the fact considered to be tough is the reproducible capacity of HUVECs and hCMVECs in a great amount. Alternatively, hESC-derived ECs (45,46) has been being attracted by the investigators. Caspi et al. (45) have successfully engineered vascularized cardiac tissue with tri-culture of hESC-CMs, hESC-ECs and EmFs, demonstrating large number of organized vessels similar to the addition of HUVECs. Furthermore, as the successful inducement and isolation of human induced pluripotent stem cells (hiPSCs) (47,48), hiPSC-derived ECs (49,50) have been brought into focus for the sake of patient-specific medicine. Samuel et al. (50) dramatically generation of functional blood vessels in vivo by hiPSC-derived ECs from the patients, which remarked the possibility of treatment for ischemic diseases.
Building of mature and organized microvasculature
Neovessels are destined to regress without the assistance of supportive cells to remodel and stabilize lumen (14,51), including fibroblasts (FBs), pericytes and MSCs (Figure 2A). FBs have been also regarded as an indispensable and significant component within the heart (8) and proven to be involved in to maintain the integrity of microvessels and prevent from leaking. Cardiac fibroblasts (CFs) are considered to play a pivotal role in normal cardiac homeostasis and pathological fibrosis after MI, which also have been presented to promote ECs proliferation and sprout formation (52,53). Compared to the CFs, EmFs are more likely to get available. EmFs have been shown to promote viability and proliferation of ECs and stabilize the neovessels in attempt to augment vascularization of the engineered cardiac tissue in which the mechanism would be the inducement of EmFs into mural cells and the secreted factors from EmFs, such as VEGF-A, PDGF-B, and Ang-1, and encourage the generation of basement membrane (45,54). Therefore, the combination of FBs with ECs and CMs could contribute to mature microvasculature and organized CMs structure. Further researches have focused on the ratio (55) and sequence (56) of ECs, FBs and CMs, which implied that precise ratio and pre-culture of ECs and FBs resulted in organized microvasculature and improved structure and function of engineered cardiac tissue. Iyer et al. engineered circulatory Matrigel-coated microchannels with capillary-like cords by sequential preculture of ECs, FBs and CMs, which conspicuously elucidated that FBs stabilized the vascular cords behind the seeding of ECs, and the 8% EC group (32% FBs and 60% CMs) was the optimal to possess the potential of vascularization due to the vascular cords and eventually develop into functional cardiac organoids with preferred excitation threshold (ET) (53).
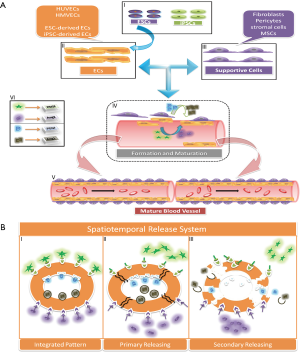
Pericyte is also naturally located in the outer part of small vessel surface, which makes tight connection with ECs through a nearly rounded cell body with numerous finger-like projections (57). Generally, pericytes detach from the surface of lumen once angiogenesis occurs, and recruit to regulate the basement membrane matrix deposition, stabilizing and maturing the newly formed vessels (14,58). Recent studies have demonstrated that perfusable microvessels could be supported and stabilized with the co-culture of ECs and pericytes (59,60). Extensively, pericytes could be also exploited into the establishment of mature perfusable microvessels within the scaffolds, providing enough microvascular niches for generation of scale-up cardiac tissue. Although advances have also been shown in vascular, skeletal, bone tissue engineering (61,62), the application of pericytes in cardiac tissue engineering is rather fewer. Several studies, however, have shown that pericytes could promote formation of microvessels and vascularization of cardiac tissue in vitro or in vivo (63,64). Moreover, human myocardial pericytes have been successfully defined and isolated (65), which probably compatible with ECs toward heart. In general, pericytes could be as a promising promoter in the combination with ECs for building more organized microvessels for thicker cardiac tissue.
In addition to the FBs and pericytes, MSCs (66,67) and stromal cells (43,68) are also viewed to be implicated in the supporting of vessel formation. Although the mechanism of auxiliary process is still clearly known, the conducive effect of supportive cells on the process of vascular maturation has been undoubtedly validated in vitro or in vivo, which can be applied in cardiac tissue engineering.
Angiogenic growth factors (AGFs)-embedded scaffolds
Considering the recent progresses of AGFs-embedded graft onto infracted zone in the neovascularization and improved cardiac function, AGFs can be employed as an effective additive in attempt to enhance the formation of new blood vessels. At present, AGFs commonly embedded within scaffolds are VEGF (25), PDGF (26), ANG-1 (69), FGF (70), and thymosin β (71). It was generally realized that the formation of neovessels was a complicated and multi-step process involved with various AGFs in different duration and phases (14), thereby VEGF alone or simple combination of AGFs cannot meet the demand of formation of robust blood vessels. Controlled release of AGFs in spatiotemporal pattern (Figure 2B), therefore, have gradually been attracted by researchers. Awada et al. designed a spatiotemporal delivery system consisted of fibrin and heparin-based coacervate (26). Specifically, fibrin scaffolds was embedded with VEGF while PDGF was loaded into the coacervate then scattered into the fibrin scaffolds, where the combined system was shown to provide a rapid release of VEGF followed by slow and sustained release of PDGF after injection into infarcted heart. Further observation demonstrated that sequential delivery of VEGF and PDGF improved overall cardiac viability and provided persistent angiogenesis in the infarcted myocardium (26). Furthermore, Brudno et al. dramatically presented a successive process of microvascular formation and vessel maturation both in vitro and in vivo (72). Temporal control of pro-angiogenic (VEGF/ANG-2) and pro-maturation (PDGF/ANG-1) factors in a macroporous polymer system could sustain and localize release of the four growth factors of interests in vivo, demonstrating high density and maturity. During the process, VEGF is an initiator to trigger ECs sprouting and proliferation to form new, immature vessel sprouts with the assistance of ANG-2, and then PDGF and ANG-1 stabilize and mature neovessels (72). Similar results have also been observed in other studies (69,73). Meanwhile, the carriers embedded with AGFs have gradually been improved and innovated in attempt to precisely control AGFs release, such microparticles (74,75), nanoscale patterns (76,77) and composite hydrogels (69,78).
Spatiotemporal release of AGFs, we believe, can be introduced into in vitro microfluidic bioreactor to sufficiently induce and augment vascularization. The reports about such controlled system applied in the heart-on-a-chip system, even so, are relatively less. Anyway, some relatively simple controlled system have been used to augment neovascularization for building thicker heart tissue in vitro (79,80). It is a long way to go, and efforts also should be continuously made to combined spatiotemporal system with microfluidic bioreactor.
Intact decellularized scaffolds
Since Ott et al. innovatively achieved an intact decellularized rat heart by retrograde coronary perfusion of detergents, preserving cardiac extracellular matrix and perfusable vascular network (28), decellularized heart have been being viewed as a perfectly circulatory scaffold to provide native microenvironment for reseeded CMs to survive and function. Admittedly, such an acellular circulatory scaffold retained precise 3D architecture, authentic ECM and inherent vasculature. Of worthy note, the retained vasculature dramatically maintained large cardiac vessels and smaller third- and fourth-level branches (28,29), which is prepared to further branch and anastomose into host vasculature. Because of the remodeled microvessels, the goal of perfusable cardiac tissue would be realized to construct thicker heart tissue in vitro.
Although decellularized heart could be a promising strategy for cardiac tissue engineering, challenges are still existed and tough. It was recognized that the ECM or microvascular composition and architecture could be disrupted during the process of decellularization. Therefore, maximizing the acellularity and minimizing the disruption of ECM and microvasculature clearly largely rely on the optimization of heart decellularization methodology, including chemical, biologic and physical techniques (30,81). The decellularized protocol currently used toward heart, to our best knowledge, is based on the comprehensive strategy, a series of multi-procedure processes. The most common used protocols were detergents (SDS, Triton-X-100) (28,82), enzyme (trypsin)-predominant (83) or both mixed method (84), which were all assisted by other factors, such as acid or solvent. Despite the extensive improvement among these methods, it was still observed that the ECM composition and structure have been damaged in varying degree. Akhyari et al. (85) compared four decellularization techniques under automatic software-operated control system in order to seek the optimal one to obtain intact acellular scaffolds, whereas none of the analyzed protocols proved as an entirely ideal option. Specifically, protocol I (SDS-based) and II (trypsin-based) showed improved preservation of glycosaminoglycan (GAG) at the cost of higher remaining DNA values, indicating cellular remnants. And only protocol I could protect collagen type IV and laminin, basement proteins, compared to other three protocols in despite of reduction in quantity relative to native heart. On contrary, protocols III (SDS- and acid-based) and IV (saponin-based) both presented lower DNA content, desirable outcome of cell remove, but protocol III showed decreased content of laminin and elastin and protocol IV showed lower GAG and laminin content. Consequently, an excellent protocol is urgently needed for the purpose of intact decellularized scaffolds. To the end, some investigators have been retrofitting the employed methods. Lee et al. presented that inverted orientation of treated heart efficiently remove cellular debris and maintain ECM integrity under the real-time monitoring of flow dynamics (86).
Another issue that should be concerned about is the efficiency of recellularization. The fact presented by previous studies was that the dispersion of seeded cells could not uniformly achieve throughout the whole heart, resulting in varying cell density (28,87), thereby the mechanical force and electrical synchronization have been affected (84). Therefore, optimal reseeding strategies also should be employed to promote the dispersion of cells uniformly. Moreover, given the complicity of the whole decellularized scaffold, some investigators pursued simple strategy, using part of decellularized scaffold to reconstruct cardiac patches in vitro for transplantation (88,89). The biggest hurdle in the field is the dearth of human donor for decellularization, restraining the clinical translation, but the xenografts, rat or porcine, were also shown to be enough to replace human heart for decellularization. Collectively, the decellularized scaffolds hold a promise for building perfusable cardiac tissue in theory, but the breakthrough among the decellularization methodology, reseeded strategy and the source of donor should be made.
Precise design of pro-angiogenic scaffolds
In terms of the high density and organic alignment of microvessels in native heart, orchestrating precise microstructure to promote and direct neovascularization is also needed to generate spatially defined and functional microvasculature, enhancing engineered tissue integration and function (90). Such scaffolds were well defined as pro-angiogenic scaffolds. For this purpose, lots of studies have been done to consummate the design of pro-angiogenic scaffolds, including porous, microchanneled, electrospun nanofibrous and bioprinted scaffolds, discussed below.
Porous scaffolds
Porous scaffolds have been widely employed in tissue engineering due to the desirable potential of cell attachment, retention and proliferation, and the highly efficient transport of oxygen, nutrition and wastes (91,92). Interestingly, investigators also found that the design of porous pattern could induce blood vessels in-growth to vascularize itself. The mostly attractive porous scaffold, to date, is the inverse opal scaffold (93,94). Compared to other methods (95), the superior merit of the inverse opal scaffold is the precisely controlled and uniform interconnected pores, providing an advantageous microenvironment for neovascularization (31,96). The study has verified that neovasculature could invade into the center of inverse opal scaffolds and augment over time by the direct non-invasive methods (96). In addition, investigators compared the effect of pore size on the potential of neovascularization, which presented different patterns of vessel formation-small vessels at high densities and poor penetration depth for scaffolds with small pores (<200 µm), and large vessels at low densities and deep penetration depth for scaffolds with large pores (>200 µm) (97). Despite of uncertainty about how pore size affects the formation of blood vessels, to date, porous scaffolds show great affinity for ECs to in-grow in. Based on the results, porous scaffolds fabricated from inverse opal scaffolds have been used in cardiac tissue engineering, resulting in conductive effect on the neovascularization (32,98). A composite scaffold was fabricated and characterized by parallel channels and micrometer-sized, interconnected pores (98). The authors revealed that CMs survived and proliferated for 2 weeks in the parallel channels in vitro and angiogenesis occurred in the interconnected pores in vivo (98). Moreover, CMs, ECs and fibroblasts were co-seeded into such composite scaffolds, and it was elucidated that such scaffolds could support seeded cells attachment and survival and induce ECs to form blood vessels (32), demonstrating the beneficial effect of porous structure on the vascularization and building of cardiac tissues. Recently, porous membranes have been used as interface membrane between two compartments due to effective permeability, discussed in the final part. Alternatively, grid scaffolds with short and long strut also shown desirable porous property and pore-interconnectivity pattern by soft lithographic and stacking technique, which have shown ECs were functional and self-assembly into vascular networks around the pores (99,100). In a word, porous scaffolds can be viewed as a great candidate for cardiac tissue engineering.
Microchanneled scaffolds
Since microfluidic scaffolds were designed and assembled, it was widely used in tissue engineering due to quick convective and diffusive mass transfer, including cardiac tissue engineering (101). Acellular microchanneled scaffolds embedded with basic fibroblast growth factor (bFGF) in a diameter of 200 µm were implanted subcutaneously in mice, observing greater vessel density compared with non-channeled scaffolds (33). Thus, microchanneled scaffolds properly served as a structural promoter to induce vascularization, and also a platform embedded with AGFs to deeply form microvessels. Furthermore, ECs and perivascular cells have been added and lined through the microchannels to form mature vascular vessels. Typically, Zheng et al. have successfully engineered integrated microvessel networks in vitro within type I collagen microchannels by addition of HUVECs and AGFs (VEGF and bFGF), which suggested that the microchanneled scaffold expressed great affinity for ECs to attach and line to form vascular networks, and communicate with perivascular cells (20). Further measurement demonstrated that such microvessel networks presented great integrality, proper permeability and long-term stability (20). Despite of advances in microchanneled scaffolds, it is still hard to mimic native microvessels due to its complex configuration and cell-by-cell communication. Even it is hard to generate effective microvascular networks within the heart-on-a-chip system in vitro (102,103), which limited the viability of heart cells. Therefore, further efforts should urgently be made to enhance endothelialization and complex configuration to achieve real vascular networks in vitro to nourish heart cells. Recently, microchanneled scaffolds have been fabricated by electrospinning and bioprinting technique, which could promote, to some extent, formation of microvascular networks and its complexity, discussed in next two parts. Collectively, microchanneled scaffold, anyway, is an excellent pro-angiogenic scaffold to promote vascularization.
Electrospun nanofibrous scaffolds
Owing to increased understanding of nanofibrous architecture of ECM in the native heart—a highly aligned and striated nanostructure to orientate CMs and enhance contractility (104,105) electrospinning technique has been commonly applied to construct nanofibrous scaffolds to mimic natural microenvironment of heart (34,106). Several studies have been made to present the fact that aligned electrospun nanofibers contributed to sarcomere formation and enhanced contractivity (35,107). Recent researches, additionally, have indicated the conductive effectiveness of fibrous scaffolds on the invading or retaining of vascular cells to form blood vessels (108,109). Tillman et al. electrospun nanofibrous polycaprolactone-collagen (PCL/collagen) scaffolds with tubular structure, which were lined with ECs and SMCs for in vivo evaluation of stability (109). In vivo observation indicated that such seeded scaffolds could support adherence and growth of ECs and SMCs and resist platelet adherence (109). Similarly, Shalumon et al. also showed that topographically aligned nanofibrous scaffolds could result in ECs attachment and organization into blood vessels (110). Obviously, ECs show preference to the topographic surface of nanofibers. Based on the tendency, investigators combined some agents with nanofibers, such as AGFs (111) and peptides (112), demonstrating synergistic effect of the integration on the superior ECs viability and high ECs and vessel density compared to nanofibers alone. Admittedly, the AGFs, such as VEGF or bFGF, play a pivotal role on the formation of blood vessels, thereby incorporation of AGFs, we believe, can serve as a beneficial strategy to amplify angiogenic effect with topography of nanofibers.
Bioprinted scaffolds
Bioprinting has been emerging as a promising technique to revolutionize medicine, widespread use in tissue engineering, including cardiac and vascular tissue engineering (113,114). The predominant advantages of 3D printing technique are involved in the art of precisely controlled structure, flexibility, scalability and reproducibility. Bioprinted scaffolds, thus, are architected in specific and orderly patterns according to researchers’ desires (115). Focuses attracted by investigators, presented in other reviews (37,114), are bioprinting methods and types of bioinks for printing 3D blood vessels, for the different choices and combination of both can lead to varied extent of vascularization. Herein, we directionally emphasize several bioprinted patterns of pro-angiogenic scaffolds to meet the needs of prevascularization.
On the one hand, direct printing technique has been widely popularized, and several vessel-like structures have been directly printed (116,117). Zhang et al. (116) bioprinted alginate hollow filaments by coaxial nozzle printers, which prolonged or assembled into microfluidic networks (117). Such alginate conduits showed great physical, mechanical and biomedical properties, especially expressing favourable biocompatibility for seeded cells (118). Although the inner and outer diameter could be adjusted through changing the flow rate of solution, the material concentration and the nozzle diameter, the ultimate diameter could not reach the capillary-scale. One concept about this is to induce capillaries formation from existing microvascular vessels, based on the mechanism of angiogenesis and vasculogenesis (21). Despite of advances about this method, the geometry and density of capillaries cannot be achieved. Thus, direct printing of capillaries is urgently needed. Recently, the application of the blend bioinks are gradually attracted by investigators, which integrated biological, biomedical and mechanical properties, even including cells. For example, Jia et al. designed a cell-responsive bioink, consisted of gelatin methacryloyl (GelMA), sodium alginate, and 4-arm poly(-ethylene glycol)-tetra-acrylate (PEGTA), to create 3D vascular networks with different aspect ratios of internal grids and numbers of layers (40). This blend bioink showed great compatibility for ECs and resulted in integrated perfusable microvascular networks, suggesting the synergistic effect of blend bioinks. Interestingly, cells have been added into bioinks, cell-laden bioinks, to print microvessels (119,120), which could exclude in vitro perfusion of ECs or seeded cells and enhance ECs lining and viability. Zhu et al. used ECs-laden bioinks to create endothelial networks by a rapid bioprinting method-microscale continuous optical bioprinting (µCOB), showing excellent anastomosis with host circulation in mice (119).
On the other hand, fugitive inks have always been considered as a paradigm tool as to indirect printing technique. About the process, fugitive inks are firstly templated into specific models and then coated by gel or synthetic materials, followed by the remove of inner inks to obtain the tubular vessel-like structures. Sacrificial carbohydrate glass, a kind of fugitive materials, was printed into vascular templates that dissolved after perfusable channels were fabricated by gel encapsulation (121). After perfusion of ECs, the author found that ECs quickly lined the inner wall and sprouted from in situ, and showed the great capacity of functional mass transport to ambient cells, even oxygen-sensitive hepatocytes (121). Moreover, Kolesky et al. developed an aqueous fugitive ink, composed of Pluronic F127, to print three-scale scaffolds, including 1D, 2D, 3D configuration, which all presented great affinity for ECs attachment and encompassment into blood vessels (120). Combined with cell-laden ECM, F123-involved scaffolds were established into a vascularized tissue structure after fugitive inks liquefied under the condition below 4 °C (120). Furthermore, they elegantly innovated the design of scaffolds, enabling to thicken the tissue and prolong the perfusable durations (122). Such fugitive inks used in 3D printing could build tubular structures easily and precisely, which gradually has been well developed and attracted.
Heart-on-a-chip: microfluidic bioreactor
Organ-on-a-chip recently has been popularized as a micro-engineered biomimetic system, aiming to finely recapitulate the organ-specific multi-cellular microstructure, tissue-tissue interfaces, physical and biochemical microenvironments and vascular perfusion (123). Various organs have been miniaturized on a chip, such as heart (124), lung (125), kidney (126), liver (127), gut (128) and so on, to deepen or broaden the research for organ regeneration, drug screening and physiological or disease models. Of worthy note, heart-on-a-chip system (Figure 3) is a versatile microfluidic bioreactor, known as miniaturized beating heart, which is designed to recapitulate the hierarchical architecture and native physiological microenvironments of its in vivo counterpart.
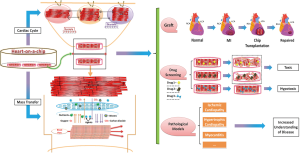
Hierarchical architecture by layer-by-layer technique
Microfluidic channels are the core of heart-on-a-chip system. Continuous perfused microchannels with or without endothelialization are conceived to resemble the microvessels in native myocardium, providing oxygen and nutrients to the heart cells and carrying metabolic wastes away (101,123). Other elements in the heart-on-a-chip system are optionally involved in the contents we concluded above, including vascular cells, AGFs and pro-angiogenic scaffolds. Using layer-by-layer technique, microfluidic bioreactor, so called heart-on-a-chip, has been assembled.
For instance, Ye et al. created a biodegradable perfusable bioreactor comprised of distinct parenchymal and vascular compartments separated by a permeable membrane interface, demonstrating the sufficient permeability of in-between interface, efficient differentiation and viability of human skeletal muscle cells in vitro, and biodegradation of the interface membrane with infiltration of host blood cells in vivo (129). Based on these results above, they artfully introduced multi-layers through-pores scaffolds and porous interface, assembled with perfusable microvessels to increase mass transfer and heart cell retention (102). More specifically, by pairwise stacking of microvessels and heart cell scaffolds in a parallel configuration, the four layer (4L) devices comprised of a microfluidic base, vascular-parenchymal interface and 2L heart cell scaffold. The novelties are incorporation of a rapidly degrading poly (glycerol sebacate) (PGS) porous interface and a more slowly degrading polymer, ammonium peroxydisulfate (APS) (130), to construct upper scaffold and base. The different rate of degradation is more precise to adapt to match the process of infracted heart healing, leading to a closer step to in vivo heart on a chip to repair of damaged heart. After four days of in vitro culture, they found that heart cells were attached and distributed throughout the internal pore architecture of the 2L heart cell scaffold (102). In this work, they demonstrated the potential of scalable unit for fabricating heart tissue, but the mass transfer in this unit was still confined and the endothelialization of channels was hard to realize.
For this goal, Morgan et al. dramatically innovated the scalable unit. They built multi-material polymer scaffolds with hierarchical pore architectures, primary (macroscale) and secondary (microscale) pores, by combining two surface-eroding elastomers, PLT32i and PGS (103). In comparison with the design mentioned above, the new system had several advantages: (I) the upper scaffolds and base made of PLT32i, a derivative of poly(limonene thioether) (PLT32o), which has proven to prolonged in vivo maintenance of 3D structural integrity and elastomeric mechanical behavior (103,131); (II) the two-scale pores architectures within upper scaffolds, primary (macroscale) and secondary (microscale) pores, which has been elucidated that the primary pores guide heart cell alignment and enable robust perfusion while the secondary pores increase heart cell retention and reduce polymer volume fraction; (III) the co-culture of HUVECs and heart cells, demonstrating spatial organization and retention of co-culture vascular cells and heart cells (103). Excitingly, the innovated design has made a further step forward the goal of prevascularized cardiac tissues to augment the thickness of engineered heart tissue in vitro, and then assembled into a heart-on-a-chip for implantation.
Furthermore, Fleischer et al. developed a comprehensive bioreactor based on the electrospinning technology, which were involved in three layers: (I) microgroove scaffolds to promote alignment of heart cells; (II) microtunnels to reside ECs with VEGF-embedded microparticles in a controlled release; (III) microcage-like scaffolds embedded with poly (lactic-co-glycolic acid) (PLGA) microparticles to control the release of anti-inflammatory drugs into the ambient microenvironment (80). Each layer was expected to play an individual pivotal role in the process of building vascularized cardiac tissue. After transplantation in vivo, the heart chip manifested excellent aligned and elongated cardiac bundles, endothelialization of microtunnels and high density of blood vessels under the controlled release of VEGF (80).
Mechanical and electrical environment
It is acknowledged that the heart is an electro-mechanical coupled living organ. With the electrical stimulus from cardiac conduction system, cardiac myocytes exhibited synchronous contraction, causing the cyclic mechanical loading to cardiac tissue to sustain homeostasis and efficient functioning. In vitro studies have shown that proper mechanical (132,133) and electrical stimulus (134,135) could contribute to differentiation and maturation of heart cells, eventually performing efficient synchronous contraction (136). To date, numerous studies introduced mechanical and electrical stimulus into microfluidic bioreactor to promote the development of heart-on-a-chip system (137). Cyclic mechanical stimulation was usually employed (124,138). Marsano et al. developed an innovative microfluidic platform stimulated by uniaxial cyclic strain, presenting superior cardiac differentiation, early spontaneous synchronous beating and better contractile capability (124). As to such bioreactor in details, the system contained two layers separated by the PDMS membrane, which the top layer was compartmentalized by two rows of hanging posts into three channels, central part filled with fibrin gel embedded cardiac cells and two lateral channels perfused with culture medium, and the bottom layer was connected to an electro-pneumatic controller as an actuation compartment. Once pressure signal was released, the middle membrane deformed and abutted onto the posts ends, and then returned previous state (124). Under the cyclic mechanical pressure, mimicking the physiological systole and diastole, the features of seeded cells show great similarities to the native counterpart. Moreover, electrical stimulus also has served as great potential to build mature cardiac tissue combined with microfluidic channels (139,140). Xiao et al. microfabricated a perfusable biowire to test pharmacological agents (139). The bioreactor comprised of a drug reservoir, a perfusable biowire (141) and a channel connecting to external peristaltic pump. The study found that CMs in the parallel- and perpendicular-stimulated biowires showed stronger expression of Cx43 compared to the control biowires, indicating better coupling between the CMs in the stimulated groups. In addition, the simutaneous performance of medium perfusion and electrical stimulation dramatically resulted in better performance. Furthermore, simultaneous mechanical and electrical stimulation have also been presented (142,143). Obviously, the comprehensive stimulation could promote maturation and contractivity.
Conclusions
In summary, we generally introduce recent accomplishments in establishing circulatory scaffolds, and present its application in cardiac bioreactors, so called heart-on-a-chip systems. In order to overcome the limitation of thickness, ECs and AGF have been added and combined with natural or synthetic biomaterials to generated circulatory scaffolds, included decellularized heart, to build and augment cardiac tissue. Moreover, heart-on-a-chip is considered as a versatile bioreactor to further recapitulate cardiac hierarchical architecture and its native physiological microenvironments to broaden the research for building cardiac tissue.
Future and direction
Although advanced progresses in the design, fabrication and assembly of circulatory scaffolds, the aim to engineer spontaneously perfusable beating cardiac tissue in vitro is still challenging and elusive. Different parts in the heart-on-a-chip systems serve as individual pivotal role to support the system functioning. Circulatory scaffold is the cornerstone of such bioreactor, which is also the tough one to overcome. For this goal, many aspects must be considered. Firstly, the proper source of ECs must be provided. While some results have been achieved, ESC-derived and iPSC-derived ECs are, recently, deeply investigated, and we believe these two sources probably present promising future due to the absence of immune ejection. Moreover, the mechanism of ECs with other supportive cells should be further explored in order to generate more mature microvascular networks. Secondly, the addition of AGFs is an efficient method to promote vascularization, and the choice of AGFs and the patterns of spatiotemporal release should also be further studied. Thirdly, the decellularized scaffolds are truly native platform for cells to attach and survive, but the xenograft is the most severe challenge. But the technological refresh of decellularization might reduce the heterogenicity and further mitigate the deconstruction of native matrix. Finally, the design of pro-angiogenic scaffolds is also important for circulatory scaffolds. Microchannels, we believe, probably are the optional pattern by several techniques, such photo or soft lithography, electrospinning and 3D printing. The pattern and feature of microchannels should be further designed and tested in order to greatly function. And how to efficiently endothelialize the microchannels must be also deeply studied in term of in vivo inosculation and perfusion. 3D printing is a great technique to precisely control geometry of scaffolds and show excellent flexibility, scalability and reproducibility. The perfect result is to effectively endothelialize the microchannels to build a stable microvasculature by cell-laden inks, which can contribute to the desire for perfusable beating heart. Recently, exosomes derived from stem cells have been applied to improve cardiac function after MI due to its promoter of vascularization and the regulator of cardiac remodeling (144), which can be, we believe, introduced into scaffolds to contribute to establish circulatory scaffolds.
Besides the circulator scaffolds, the assembly of heart-on-a-chip system is also a pivotal role. Layer-by-layer is the common technique in the process, which assembles individual functioning layer to make a sufficient microfluidic bioreactor. Heart cells layers should be mature and anisotropic, even beat spontaneously and synchronously under the capacity of quick mass transfer from circulatory scaffolds. Moreover, the pattern of assembly should be also considered. An opinion about the gradual transition of orientation within the multi-layered cardiac muscle has been proposed as a direction to mimic native heart tissue (80,106). For these above, we still have a long way to go.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81671832 and 81571826).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation 2017;135:e146-e603. [Crossref] [PubMed]
- Laflamme MA, Murry CE. Heart regeneration. Nature 2011;473:326-35. [Crossref] [PubMed]
- Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol 2012;28:719-41. [Crossref] [PubMed]
- Yacoub M. Cardiac donation after circulatory death: a time to reflect. Lancet 2015;385:2554-6. [Crossref] [PubMed]
- Zandonella C. Tissue engineering: the beat goes on. Nature 2003;421:884-6. [Crossref] [PubMed]
- Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng 2009;11:289-313. [Crossref] [PubMed]
- Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res 2016;118:400-9. [Crossref] [PubMed]
- Radisic M, Deen W, Langer R, et al. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol 2005;288:H1278-89. [Crossref] [PubMed]
- Sun X, Altalhi W, Nunes SS. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv Drug Deliv Rev 2016;96:183-94. [Crossref] [PubMed]
- Akintewe OO, Roberts EG, Rim NG, et al. Design approaches to myocardial and vascular tissue engineering. Annu Rev Biomed Eng 2017;19:389-414. [Crossref] [PubMed]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995;11:73-91. [Crossref] [PubMed]
- Risau W. Mechanisms of angiogenesis. Nature 1997;386:671-4. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. [Crossref] [PubMed]
- Ribatti D, Vacca A, Nico B, et al. Postnatal vasculogenesis. Mech Dev 2001;100:157-63. [Crossref] [PubMed]
- Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nat Med 1997;3:158-64. [Crossref] [PubMed]
- Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016;119:91-112. [Crossref] [PubMed]
- Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal 2006;8:1907-39. [Crossref] [PubMed]
- Rodriguez-Porcel M. Non-invasive monitoring of angiogenesis in cardiology. Curr Cardiovasc Imaging Rep 2009;2:59-66. [Crossref] [PubMed]
- Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 2012;109:9342-7. [Crossref] [PubMed]
- Lee VK, Lanzi AM, Haygan N, et al. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng 2014;7:460-72. [Crossref] [PubMed]
- Radisic M, Malda J, Epping E, et al. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng 2006;93:332-43. [Crossref] [PubMed]
- Korecky B, Hai CM, Rakusan K. Functional capillary density in normal and transplanted rat hearts. Can J Physiol Pharmacol 1982;60:23-32. [Crossref] [PubMed]
- Kim JJ, Hou L, Huang NF. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater 2016;41:17-26. [Crossref] [PubMed]
- Rufaihah AJ, Vaibavi SR, Plotkin M, et al. Enhanced infarct stabilization and neovascularization mediated by VEGF-loaded PEGylated fibrinogen hydrogel in a rodent myocardial infarction model. Biomaterials 2013;34:8195-202. [Crossref] [PubMed]
- Awada HK, Johnson NR, Wang Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction. J Control Release 2015;207:7-17. [Crossref] [PubMed]
- Rodness J, Mihic A, Miyagi Y, et al. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater 2016;45:169-81. [Crossref] [PubMed]
- Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008;14:213-21. [Crossref] [PubMed]
- Sarig U, Au-Yeung GC, Wang Y, et al. Thick acellular heart extracellular matrix with inherent vasculature: a potential platform for myocardial tissue regeneration. Tissue Eng Part A 2012;18:2125-37. [Crossref] [PubMed]
- Zia S, Mozafari M, Natasha G, et al. Hearts beating through decellularized scaffolds: whole-organ engineering for cardiac regeneration and transplantation. Crit Rev Biotechnol 2016;36:705-15. [PubMed]
- Zhang YS, Zhu C, Xia Y. Inverse opal scaffolds and their biomedical applications. Adv Mater 2017.29. [PubMed]
- Thomson KS, Korte FS, Giachelli CM, et al. Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng Part A 2013;19:967-77. [Crossref] [PubMed]
- Zieber L, Or S, Ruvinov E, et al. Microfabrication of channel arrays promotes vessel-like network formation in cardiac cell construct and vascularization in vivo. Biofabrication 2014;6. [Crossref] [PubMed]
- Peng S, Jin G, Li L, et al. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem Soc Rev 2016;45:1225-41. [Crossref] [PubMed]
- Guex AG, Frobert A, Valentin J, et al. Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomater 2014;10:2996-3006. [Crossref] [PubMed]
- Kharaziha M, Nikkhah M, Shin SR, et al. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013;34:6355-66. [Crossref] [PubMed]
- Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016;34:422-34. [Crossref] [PubMed]
- Datta P, Ayan B, Ozbolat IT. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater 2017;51:1-20. [Crossref] [PubMed]
- Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol 2005;23:879-84. [Crossref] [PubMed]
- Jia W, Gungor-Ozkerim PS, Zhang YS, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016;106:58-68. [Crossref] [PubMed]
- Narmoneva DA, Vukmirovic R, Davis ME, et al. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 2004;110:962-8. [Crossref] [PubMed]
- Lesman A, Habib M, Caspi O, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A 2010;16:115-25. [Crossref] [PubMed]
- Tulloch NL, Muskheli V, Razumova MV, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 2011;109:47-59. [Crossref] [PubMed]
- Valarmathi MT, Fuseler JW, Davis JM, et al. A novel human tissue-engineered 3-D functional vascularized cardiac muscle construct. Front Cell Dev Biol 2017;5:2. [Crossref] [PubMed]
- Caspi O, Lesman A, Basevitch Y, et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 2007;100:263-72. [Crossref] [PubMed]
- Giacomelli E, Bellin M, Sala L, et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017;144:1008-17. [Crossref] [PubMed]
- Zaehres H, Scholer HR. Induction of pluripotency: from mouse to human. Cell 2007;131:834-5. [Crossref] [PubMed]
- Papapetrou EP. Induced pluripotent stem cells, past and future. Science 2016;353:991-2. [Crossref] [PubMed]
- Yue X, Acun A, Zorlutuna P. Transcriptome profiling of 3D co-cultured cardiomyocytes and endothelial cells under oxidative stress using a photocrosslinkable hydrogel system. Acta Biomater 2017;58:337-48. [Crossref] [PubMed]
- Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A 2013;110:12774-9. [Crossref] [PubMed]
- Coulombe KL, Bajpai VK, Andreadis ST, et al. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng 2014;16:1-28. [Crossref] [PubMed]
- Twardowski RL, Black LD 3rd. Cardiac fibroblasts support endothelial cell proliferation and sprout formation but not the development of multicellular sprouts in a fibrin gel co-culture model. Ann Biomed Eng 2014;42:1074-84. [Crossref] [PubMed]
- Iyer RK, Chiu LL, Vunjak-Novakovic G, et al. Biofabrication enables efficient interrogation and optimization of sequential culture of endothelial cells, fibroblasts and cardiomyocytes for formation of vascular cords in cardiac tissue engineering. Biofabrication 2012;4. [Crossref] [PubMed]
- He W, Ye L, Li S, et al. Construction of vascularized cardiac tissue from genetically modified mouse embryonic stem cells. J Heart Lung Transplant 2012;31:204-12. [Crossref] [PubMed]
- Iyer RK, Chui J, Radisic M. Spatiotemporal tracking of cells in tissue-engineered cardiac organoids. J Tissue Eng Regen Med 2009;3:196-207. [Crossref] [PubMed]
- Iyer RK, Chiu LL, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A 2009;89:616-31. [Crossref] [PubMed]
- Murray IR, Baily JE, Chen WC, et al. Skeletal and cardiac muscle pericytes: functions and therapeutic potential. Pharmacol Ther 2017;171:65-74. [Crossref] [PubMed]
- Stratman AN, Malotte KM, Mahan RD, et al. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009;114:5091-101. [Crossref] [PubMed]
- van der Meer AD, Orlova VV, ten Dijke P, et al. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 2013;13:3562-8. [Crossref] [PubMed]
- Kim J, Chung M, Kim S, et al. Engineering of a biomimetic pericyte-covered 3D microvascular network. PLoS One 2015;10. [Crossref] [PubMed]
- Avolio E, Alvino VV, Ghorbel MT, et al. Perivascular cells and tissue engineering: current applications and untapped potential. Pharmacol Ther 2017;171:83-92. [Crossref] [PubMed]
- Gökçinar-Yagci B, Uckan-Cetinkaya D, Celebi-Saltik B. Pericytes: properties, functions and applications in tissue engineering. Stem Cell Rev 2015;11:549-59. [Crossref] [PubMed]
- Riemenschneider SB, Mattia DJ, Wendel JS, et al. Inosculation and perfusion of pre-vascularized tissue patches containing aligned human microvessels after myocardial infarction. Biomaterials 2016;97:51-61. [Crossref] [PubMed]
- Schaefer JA, Guzman PA, Riemenschneider SB, et al. A cardiac patch from aligned microvessel and cardiomyocyte patches. J Tissue Eng Regen Med 2018;12:546-56. [Crossref] [PubMed]
- Chen WC, Baily JE, Corselli M, et al. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells 2015;33:557-73. [Crossref] [PubMed]
- Valarmathi MT, Fuseler JW, Potts JD, et al. Functional tissue engineering: a prevascularized cardiac muscle construct for validating human mesenchymal stem cells engraftment potential in vitro. Tissue Eng Part A 2018;24:157-85. [Crossref] [PubMed]
- Mishra R, Roux BM, Posukonis M, et al. Effect of prevascularization on in vivo vascularization of poly(propylene fumarate)/fibrin scaffolds. Biomaterials 2016;77:255-66. [Crossref] [PubMed]
- Roberts MA, Tran D, Coulombe KL, et al. Stromal cells in dense collagen promote cardiomyocyte and microvascular patterning in engineered human heart tissue. Tissue Eng Part A 2016;22:633-44. [Crossref] [PubMed]
- Rufaihah AJ, Johari NA, Vaibavi SR, et al. Dual delivery of VEGF and ANG-1 in ischemic hearts using an injectable hydrogel. Acta Biomater 2017;48:58-67. [Crossref] [PubMed]
- Chu H, Chen CW, Huard J, et al. The effect of a heparin-based coacervate of fibroblast growth factor-2 on scarring in the infarcted myocardium. Biomaterials 2013;34:1747-56. [Crossref] [PubMed]
- Chiu LL, Radisic M. Controlled release of thymosin beta4 using collagen-chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. J Control Release 2011;155:376-85. [Crossref] [PubMed]
- Brudno Y, Ennett-Shepard AB, Chen RR, et al. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 2013;34:9201-9. [Crossref] [PubMed]
- Kim JH, Jung Y, Kim SH, et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 2011;32:6080-8. [Crossref] [PubMed]
- Formiga FR, Pelacho B, Garbayo E, et al. Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. J Control Release 2014;173:132-9. [Crossref] [PubMed]
- Pascual-Gil S, Simon-Yarza T, Garbayo E, et al. Tracking the in vivo release of bioactive NRG from PLGA and PEG-PLGA microparticles in infarcted hearts. J Control Release 2015;220:388-96. [Crossref] [PubMed]
- Izadifar M, Kelly ME, Chen X. Regulation of sequential release of growth factors using bilayer polymeric nanoparticles for cardiac tissue engineering. Nanomedicine (Lond) 2016;11:3237-59. [Crossref] [PubMed]
- Izadifar M, Haddadi A, Chen X, et al. Rate-programming of nano-particulate delivery systems for smart bioactive scaffolds in tissue engineering. Nanotechnology 2015;26. [Crossref] [PubMed]
- Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv 2013;10:59-72. [Crossref] [PubMed]
- Chung HJ, Kim JT, Kim HJ, et al. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release 2015;205:218-30. [Crossref] [PubMed]
- Fleischer S, Shapira A, Feiner R, et al. Modular assembly of thick multifunctional cardiac patches. Proc Natl Acad Sci U S A 2017;114:1898-903. [Crossref] [PubMed]
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233-43. [Crossref] [PubMed]
- Sánchez PL, Fernandez-Santos ME, Costanza S, et al. Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials 2015;61:279-89. [Crossref] [PubMed]
- Wainwright JM, Czajka CA, Patel UB, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 2010;16:525-32. [Crossref] [PubMed]
- Lu TY, Lin B, Kim J, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun 2013;4:2307. [Crossref] [PubMed]
- Akhyari P, Aubin H, Gwanmesia P, et al. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods 2011;17:915-26. [Crossref] [PubMed]
- Lee PF, Chau E, Cabello R, et al. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater 2017;49:181-91. [Crossref] [PubMed]
- Ng SL, Narayanan K, Gao S, et al. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials 2011;32:7571-80. [Crossref] [PubMed]
- Godier-Furnémont AF, Martens TP, Koeckert MS, et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A 2011;108:7974-9. [Crossref] [PubMed]
- Sarig U, Nguyen EB, Wang Y, et al. Pushing the envelope in tissue engineering: ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng Part A 2015;21:1507-19. [Crossref] [PubMed]
- Baranski JD, Chaturvedi RR, Stevens KR, et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A 2013;110:7586-91. [Crossref] [PubMed]
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater 2005;4:518-24. [Crossref] [PubMed]
- Engelmayr GC Jr, Cheng M, Bettinger CJ, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater 2008;7:1003-10. [Crossref] [PubMed]
- Zhang YS, Choi S-W, Xia Y. Inverse opal scaffolds for applications in regenerative medicine. Soft Matter 2013;9:9747-54. [Crossref]
- Zhang YS, Regan KP, Xia Y. Controlling the pore sizes and related properties of inverse opal scaffolds for tissue engineering applications. Macromol Rapid Commun 2013;34:485-91. [Crossref] [PubMed]
- Janik H, Marzec M. A review: fabrication of porous polyurethane scaffolds. Mater Sci Eng C Mater Biol Appl 2015;48:586-91. [Crossref] [PubMed]
- Cai X, Zhang Y, Li L, et al. Investigation of neovascularization in three-dimensional porous scaffolds in vivo by a combination of multiscale photoacoustic microscopy and optical coherence tomography. Tissue Eng Part C Methods 2013;19:196-204. [Crossref] [PubMed]
- Choi SW, Zhang Y, Macewan MR, et al. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv Healthc Mater 2013;2:145-54. [Crossref] [PubMed]
- Madden LR, Mortisen DJ, Sussman EM, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 2010;107:15211-6. [Crossref] [PubMed]
- Kolewe ME, Park H, Gray C, et al. 3D structural patterns in scalable, elastomeric scaffolds guide engineered tissue architecture. Adv Mater 2013;25:4459-65. [Crossref] [PubMed]
- Lee BW, Liu B, Pluchinsky A, et al. Modular assembly approach to engineer geometrically precise cardiovascular tissue. Adv Healthc Mater 2016;5:900-6. [Crossref] [PubMed]
- Choi NW, Cabodi M, Held B, et al. Microfluidic scaffolds for tissue engineering. Nat Mater 2007;6:908-15. [Crossref] [PubMed]
- Ye X, Lu L, Kolewe ME, et al. Scalable units for building cardiac tissue. Adv Mater 2014;26:7202-8. [Crossref] [PubMed]
- Morgan KY, Sklaviadis D, Tochka ZL, et al. Multi-material tissue engineering scaffold with hierarchical pore architecture. Adv Funct Mater 2016;26:5873-83. [Crossref] [PubMed]
- Pope AJ, Sands GB, Smaill BH, et al. Three-dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol 2008;295:H1243-H1252. [Crossref] [PubMed]
- Stephenson RS, Agger P, Lunkenheimer PP, et al. The functional architecture of skeletal compared to cardiac musculature: myocyte orientation, lamellar unit morphology, and the helical ventricular myocardial band. Clin Anat 2016;29:316-32. [Crossref] [PubMed]
- Wu Y, Wang L, Guo B, et al. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 2017;11:5646-59. [Crossref] [PubMed]
- Capulli AK, MacQueen LA, Sheehy SP, et al. Fibrous scaffolds for building hearts and heart parts. Adv Drug Deliv Rev 2016;96:83-102. [Crossref] [PubMed]
- Wong HK, Ivan Lam CR, Wen F, et al. Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers. Biofabrication 2016;8. [Crossref] [PubMed]
- Tillman BW, Yazdani SK, Lee SJ, et al. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009;30:583-8. [Crossref] [PubMed]
- Shalumon KT, Deepthi S, Anupama MS, et al. Fabrication of poly (L-lactic acid)/gelatin composite tubular scaffolds for vascular tissue engineering. Int J Biol Macromol 2015;72:1048-55. [Crossref] [PubMed]
- Lakshmanan R, Kumaraswamy P, Krishnan UM, et al. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials 2016;97:176-95. [Crossref] [PubMed]
- Campagnolo P, Gormley AJ, Chow LW, et al. Pericyte seeded dual peptide scaffold with improved endothelialization for vascular graft tissue engineering. Adv Healthc Mater 2016;5:3046-55. [Crossref] [PubMed]
- Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 2015;33:395-400. [Crossref] [PubMed]
- Richards D, Jia J, Yost M, et al. 3D bioprinting for vascularized tissue fabrication. Ann Biomed Eng 2017;45:132-47. [Crossref] [PubMed]
- Derby B. Printing and prototyping of tissues and scaffolds. Science 2012;338:921-6. [Crossref] [PubMed]
- Zhang Y, Yu Y, Chen H, et al. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013;5. [Crossref] [PubMed]
- Gao Q, He Y, Fu JZ, et al. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015;61:203-15. [Crossref] [PubMed]
- Dolati F, Yu Y, Zhang Y, et al. In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 2014;25. [Crossref] [PubMed]
- Zhu W, Qu X, Zhu J, et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017;124:106-15. [Crossref] [PubMed]
- Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;26:3124-30. [Crossref] [PubMed]
- Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11:768-74. [Crossref] [PubMed]
- Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179-84. [Crossref] [PubMed]
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32:760-72. [Crossref] [PubMed]
- Marsano A, Conficconi C, Lemme M, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016;16:599-610. [Crossref] [PubMed]
- Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010;328:1662-8. [Crossref] [PubMed]
- Wilmer MJ, Ng CP, Lanz HL, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol 2016;34:156-70. [Crossref] [PubMed]
- Bavli D, Prill S, Ezra E, et al. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci U S A 2016;113:E2231-40. [Crossref] [PubMed]
- Kim HJ, Li H, Collins JJ, et al. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 2016;113:E7-15. [Crossref] [PubMed]
- Ye X, Lu L, Kolewe ME, et al. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials 2013;34:10007-15. [Crossref] [PubMed]
- Bettinger CJ, Bruggeman JP, Borenstein JT, et al. In vitro and in vivo degradation of poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) elastomers. J Biomed Mater Res A 2009;91:1077-88. [Crossref] [PubMed]
- Fischer KM, Morgan KY, Hearon K, et al. Poly(limonene thioether) scaffold for tissue engineering. Adv Healthc Mater 2016;5:813-21. [Crossref] [PubMed]
- Ruan JL, Tulloch NL, Saiget M, et al. Mechanical stress promotes maturation of human myocardium from pluripotent stem cell-derived progenitors. Stem Cells 2015;33:2148-57. [Crossref] [PubMed]
- Zhang W, Kong CW, Tong MH, et al. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: effects of niche cell supplementation and mechanical stimulation. Acta Biomater 2017;49:204-17. [Crossref] [PubMed]
- Hirt MN, Boeddinghaus J, Mitchell A, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol 2014;74:151-61. [Crossref] [PubMed]
- Heidi Au HT, Cui B, Chu ZE, et al. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip 2009;9:564-75. [Crossref] [PubMed]
- Ruan JL, Tulloch NL, Razumova MV, et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 2016;134:1557-67. [Crossref] [PubMed]
- Stoppel WL, Kaplan DL, Black LD 3rd. Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev 2016;96:135-55. [Crossref] [PubMed]
- Lux M, Andree B, Horvath T, et al. In vitro maturation of large-scale cardiac patches based on a perfusable starter matrix by cyclic mechanical stimulation. Acta Biomater 2016;30:177-87. [Crossref] [PubMed]
- Xiao Y, Zhang B, Liu H, et al. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 2014;14:869-82. [Crossref] [PubMed]
- Maidhof R, Tandon N, Lee EJ, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 2012;6:e12-23. [Crossref] [PubMed]
- Nunes SS, Miklas JW, Liu J, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 2013;10:781-7. [Crossref] [PubMed]
- Morgan KY, Black LD 3rd. Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue Eng Part A 2014;20:1654-67. [Crossref] [PubMed]
- Miklas JW, Nunes SS, Sofla A, et al. Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 2014;6. [Crossref] [PubMed]
- Jung JH, Fu X, Yang PC. Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circ Res 2017;120:407-17. [Crossref] [PubMed]