Concurrent thymic carcinoma and middle lobe syndrome
Introduction
Thymic carcinoma is the rare malignancy of thymic epithelial cells. Usually occurring in the anterior mediastinum, the tumor may spread into its surrounding tissues such as the pericardium, sternum and intra-thoracic vessels. So far, the universally recognized treatment for thymic carcinoma remains a multimodality approach including surgical resection, radiotherapy and chemotherapy, among which complete resection plays a critical role in the overall survival. Generally, according to the Masaoka-Koga staging system, thymic neoplasms of stage I and II, most stage III and some stage IV thymic neoplasms are potentially resectable (1,2). Middle lobe syndrome refers to the recurrent or chronic atelectasis of the right middle lobe of the lung. It can be classified as an obstructive type and a non-obstructive type according to whether an airway occlusion exists by bronchoscopy. For the former type, the causes which lead to the middle lobar obstruction should be found. Surgical intervention is usually indicated if neoplasms exist. The non-obstructive type also calls for surgery when conservative therapies do not work or secondary morbidities like scarring exists (3). However, in the case that one patient was diagnosed with both the non-obstructive middle lobe syndrome and thymic cancer at the same time, how to map out the best treatment plan can be a challenge: (I) it is sometimes not likely to resect the middle lobe and thymic carcinoma simultaneously, especially when the thymic carcinoma has invaded its adjacent tissues or organs; (II) if thymectomy is done first, the patient may run a high risk of postoperative pulmonary infection because of the successive secretions from the collapsed middle lobe; (III) if middle lobectomy is performed in priority, the thymic tumor may progress to unresectable disease. In this study, we reported a case who suffered from both advanced thymic carcinoma and refractory non-obstructive middle lobe syndrome, smoothly recovered from thymic carcinoma resection with no significant pulmonary infection.
Case presentation
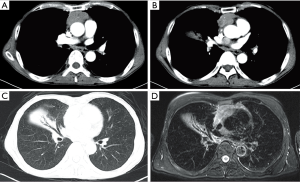
A 65-year-old male was admitted with the complaint of intermittent expectoration for more than 10 years. Before admission to our ward, he had received several treatment regimens by antibiotics in the last decade, but none of the treatments worked to decrease or stop his sputum production. The sputum was yellowish, sticky and had no unpleasant smell. The average volume of sputum reached 30–50 mL per day and most of the sputum was produced in the morning. No chest pain, dyspnea, wheezing, fever, shivering, tachycardia or myasthenia was noted. Lung auscultation revealed no rales or other abnormal findings. Both the white blood cell count and percentage of neutrophils were within the normal range. Chest enhanced computed tomography (CT) scan showed a 5.7 cm × 3.2 cm mass in the anterior mediastinum, a nodule of soft tissue between the left atrium and ascending aorta (1.9 cm), and atelectasis of the middle lobe of the right lung. The CT results were confirmed by similar manifestations in the magnetic resonance imaging (MRI) scan (Figure 1). No bronchial obstruction or stenosis but a few purulent secretions from the middle lobe were observed during the bronchoscopy. Other preoperative examinations, such as electrocardiogram, pulmonary function test, abdominal CT scan, head MRI, emission CT, yielded normal results.
Repeated sputum smear and culture were arranged for the patient after admission. He also received treatments including bronchodilators, chest physical exercise and postural sputum drainage. We dynamically observed the amount and characteristics of the sputum every 24 hours. However, the amount of purulent sputum did not decrease two weeks later. No abnormal bacteria or fungi were found by the repeated sputum culture. No antibiotics were used before the operation.
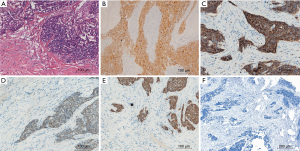
To prevent progression of the tumor, the patient received the midline sternotomy operation. We found the mediastinal tumor had invaded the mediastinal pleura and tightly adhered to the pericardium, anterior segment of the right upper lobe and the outer membrane of the ascending aorta. And those operation findings were confirmed by a pathological review. The intrapericardial nodule, which located behind the ascending aorta and beneath the right pulmonary artery, turned out unresectable for its invasion of the left atrium. The mediastinal tumor and the tumor-involved pericardium, outer membrane of the aorta, part of the right upper lobe were resected. After the operation, we arranged similar preoperative sputum examinations and supportive treatments like chest physical therapy. The patient also received therapies such as sputum aspiration by bronchofibroscope and venous antimicrobials. The recurrent cough with purulent expectoration still lasted, but no noticeable infection of the right lung occurred. Finally, he smoothly recovered and discharged at the 8th day after the operation. After pathological review, the patient was diagnosed with moderately differentiated squamous cell carcinoma of the thymus (Stage IVA according to the Masaoka-Koga staging system, Figure 2). We advised radiotherapy and platinum-based chemotherapy as postoperative treatments for the patient. During the follow-up on October 16, 2017, the patient was still alive ten months after surgery.
Discussion
Surgery remains to be the mainstay among treatments for thymic carcinoma, even for selected patients with locally advanced diseases (2,4). Pulmonary infection, which increases hospitalization costs and mortality, develops in 2.9–10.7% patients after thoracic surgery (5). Often found in elderly patients, thymic neoplasms frequently comorbid with conditions like malnutrition or compromised pulmonary function. Strict screening and treating the lung comorbidities before operation helps to reduce pulmonary complications.
External compression of the middle lobe bronchus may result in middle lobe syndrome while endobronchial lesions, such as broncholithiasis and bronchiectasis, also contribute to atelectasis of the middle lobe. Compression by tumors or their metastatic lymph nodes is considered as one of the most common causes of the middle lobe syndrome (3). We report a rare case that the non-obstructive type of the middle lobe syndrome whom complicated simultaneously by thymic carcinoma. Simultaneous surgical resection of both foci is desirable, but it is not always possible to do so due to the different anatomical positions of the middle lobe and the thymic neoplasm. Middle lobe syndrome often presents with a chronic cough with purulent expectoration, hemoptysis, pneumonia and weight loss. Because of the surgical changes after thymic tumor removal and postanesthetic effects, the secretions from the middle lobe may increase the risk of pneumonia. However, a progression of the thymic carcinoma may occur if the middle lobectomy is performed first. Under this circumstance, which disease should be treated with priority becomes a dilemma.
Pulmonary infections have long been recognized as common postoperative complications after tracheal intubation during general anesthesia. If patients ready for non-emergency operations suffer from pneumonia, they will generally receive the surgery after the infection has been effectively controlled. And it is especially the case with thoracic surgeries in which the lung is exposed to infection risks like one-lung ventilation. Our case shows that thymectomy through midline sternotomy may not necessarily lead to postoperative pneumonia even if the patient has a long-lasting expectoration symptom due to the middle lobe syndrome. If the sputum culture does not show abnormal bacteria, the patient might still recover from the operation without pulmonary complications. The case provided valuable treatment information for patients undergoing thoracotomy with pneumonia which is difficult to cure in a short time.
The risk factors which may contribute to infection after the operation should never be underestimated. Surgeons should pay special attention to elder patients with diabetes mellitus, chronic obstructive pulmonary disease or superinfection after antibiotics abuse. Close evaluation of the symptoms, physical examination, hemogram, sputum culture and lung imaging tests is warranted before and after sternotomy. Similar to antimicrobial prophylaxis in other thoracic surgeries, antibiotics such as cephalosporins and clindamycin, which cover common respiratory pathogens, may be recommended to prevent postoperative infection of the lung (6). Afterwards, doctors may choose proper antibiotics according to the sputum examinations once pulmonary infection appears. Besides, bronchodilators, postural drainage, sputum dilution therapy and aspiration of sputum may be justified. For patients with myasthenia gravis, their inability to cough and expectorate make them especially susceptible to postoperative pulmonary infection. And vice versa, infection is also associated with exacerbated myasthenia gravis. Therefore, to relieve myasthenia symptoms before operation with treatments such as corticosteroids, pyridostigmine, immunoglobulin or plasmapheresis, should always be emphasized to reduce the postoperative pneumonia (7).
In conclusion, thymic carcinoma which coexists with middle lobe syndrome is a rare condition and can be a challenge in the clinical treatments. For thymic cancer patients who have normal respiratory function and no myasthenic symptoms, the coexistent middle lobe syndrome may not improve the risk of post-thymectomy pulmonary infection if it is properly treated before the operation. When thymic carcinoma and middle lobe syndrome co-occur in the same patient, the thymic tumor might be addressed in priority to increase the chance of complete resection.
Acknowledgements
Funding: This work was supported by Science & Technology Department of Sichuan Province [2016FZ0104].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed consent: Written informed consent was obtained from the patient for publication of this manuscript and any related images.
References
- Zhao Y, Zhao H, Hu D, et al. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg 2013;96:1019-24. [Crossref] [PubMed]
- Ried M, Marx A, Götz A, et al. State of the art: diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2016;49:1545-52. [Crossref] [PubMed]
- Pejhan S, Salehi F, Niusha S, et al. Ten years' experience in surgical treatment of right middle lobe syndrome. Ann Thorac Cardiovasc Surg 2015;21:354-8. [Crossref] [PubMed]
- Shapiro M, Korst RJ. Surgical approaches for stage IVA thymic epithelial tumors. Front Oncol 2014;3:332. [Crossref] [PubMed]
- Simonsen DF, Søgaard M, Bozi I, et al. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med 2015;109:1340-6. [Crossref] [PubMed]
- Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195-283. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]


