Influence of estrogen in non-small cell lung cancer and its clinical implications
Epidemiology of non-small cell lung cancer (NSCLC)
Differences by sex and hormonal status
Lung cancer (LC) remains the leading cause of cancer-related deaths worldwide. NSCLC is the predominant type, accounting for approximately 85% of all newly diagnosed cases, with adenocarcinoma being the most common subtype of NSCLC. Although LC was an important health problem predominantly among men, in the past three decades LC incidence rates have declined about twice as fast in men as compared to women. Similarly, while LC mortality rates have decreased among men, they have increased among women. Interestingly, in 2013 LC surpassed breast cancer as the leading cause of cancer death among females in more developed countries (1,2).
It has been suggested that the increase in NSCLC incidence among women can be explained by the increased number of smoker women in developed countries (3). However, this explanation is not entirely satisfactory given that up to 53% of women who develop NSCLC were never-smokers while only 15% of men who develop NSCLC were never-smokers. Moreover, there are studies showing that among women and men with similar tobacco exposure, the onset of LC occurs earlier in women (4,5). This indicates that, in addition to smoking, there are other factors influencing the development of NSCLC in women. Furthermore, the clinical characteristics of female and male patients are very different. For instance, in females: (I) the median age at the time of diagnosis is lower than that of males; (II) there is generally no history of tobacco exposure; (III) the predominant histological subtype is adenocarcinoma; (IV) outcomes are generally better at all diagnosis stages; (V) a positive epidermal growth factor receptor (EGFR) mutation status is more common (6,7).
Several studies have shown that response to chemotherapy and survival is better in women compared to men, however these studies included almost exclusively postmenopausal women. Interestingly, when female patients are analyzed by hormonal status, premenopausal women are more commonly diagnosed with advanced stage-disease, have less differentiated tumors, a higher number of distant metastases and worse prognosis than both men and postmenopausal women (8-11).
Controversy has surrounded the issue regarding the differential incidence, clinical presentation and mortality of LC in women as compared to men. This is partly due to the difficulty in discriminating between differences that are strictly related to sex and those related to gender. Sex is a biological variable defined by differential chromosomes, genetic polymorphisms, reproductive organs, sex steroid levels, metabolism and immune responses, all of which play an important role during carcinogenesis. In contrast, gender refers to social and cultural differences in behavior and activities that can also have an effect on the course of the disease such as differential access to healthcare, health-seeking behavior, exposure to specific risk factors like smoking (12), as well as differential environmental and occupational exposures, like wood-smoke (13). In spite of this controversy, it is now beginning to be recognized that LC is a different disease in women and men, partly because of the influence of estrogen.
Influence of estrogen in outcome of NSCLC
Estrogens are pleiotropic steroid hormones involved in many physiological processes including the development of the female reproductive system, the maintenance of secondary sex characteristics as well as metabolism regulation. In addition, estrogen has also been shown to be involved in lung development and functioning in both women and men through activation of its receptor, which is widely expressed in lung epithelium (14). More recently, it has been shown that 17-β-estradiol (E2), the most potent form of estrogen, plays an important role during lung carcinogenesis.
For instance, high endogenous levels of circulating estrogen have been associated with poor survival in premenopausal women (15). Moreover, among male patients with advanced NSCLC, those with high serum levels of free β-estradiol have a significantly worse survival than those with lower β-estradiol levels (16). Therefore, estrogen status is now recognized to be associated with LC outcome in women, but interestingly, also in men.
Similarly, the association between estrogen administration and LC incidence and mortality has also been investigated. The role of hormone-replacement therapy (HRT) in development of LC is still controversial. Some studies have shown an association between HRT use and an increased incidence in LC (17-19). A prospective cohort of 36,588 peri- and postmenopausal women found that the use of combined therapy based on estrogen plus progestin increased the risk of LC in a duration-dependent manner, with an approximate 50% increased risk for 10 years of use, or longer (even after adjusting for smoking, age, and other potential confounders) (18). Nevertheless, other studies report no association between HRT use (estrogen or estrogen plus progestin) and the incidence of LC (20-22). The Women’s Health Initiative (WHI) randomized controlled trial that included 16,000 women treated with combined HRT (estrogen 0.62 mg plus progestin 2.5 mg) did not demonstrate a significant increase in LC incidence, but did show a significant increase in the number of deaths from LC compared to placebo and an absolute increase in mortality in current smokers at baseline was observed. Poorly differentiated tumors and distant metastasis were also reported in women who use combined HRT (23). The effect of combined hormone therapy on death from LC was not modified by age at screening, pervious hormone treatment, or smoking status. The increase in mortality is attenuated after cessation of hormonal therapy (24). Moreover, worse survival outcomes have been observed in women who used HRT prior to diagnosis compared to women who had never received HRT (39 vs. 79 months) (25). All these findings together support the role of estrogen in lung carcinogenesis and in LC outcome.
Aromatase expression in NSCLC and its Clinical Implication
Aromatase (ARO) is a cytochrome P-450 enzyme (CYP19A1) that mediates the final and rate-limiting step in estrogen synthesis, catalyzing conversion of testosterone to estrogen. High aromatase expression has been reported in several NSCLC cell lines and in about 44 to 86% of female and male NSCLC tissues (26-28). Niikawa and coworkers reported higher aromatase expression in lung tumor tissues compared to adjacent non-neoplastic tissues, and showed that E2 levels are higher in tumor tissue compared to healthy lung tissue, revealing that the majority of intratumor estradiol is produced locally by the activity of aromatase. Aromatase expression has not only been associated with higher estrogen levels, but also with a higher expression of estrogen receptor beta (ERβ), also known as ESR2 (29).
Moreover, aromatase expression has been detected in NSCLC metastases and, compared with primary tumors, metastatic lesions have a higher expression of aromatase, suggesting that locally synthesized estrogen may promote malignant progression, which could be controlled with aromatase inhibitors (26,30).
Further demonstrating the important role of estrogen in LC progression, aromatase overexpression has been associated with worse prognosis and poor survival in male and female patients with LC (Table 1) (27,28,31). In contrast, lower levels of aromatase are associated with greater survival in older women (>65 years old) (32), while higher intratumoral aromatase expression is associated with tumor progression via an estrogen signaling pathway in postmenopausal females with wild type EGFR lung adenocarcinoma (33). Combined ERβ and aromatase expression has been associated with poor clinical outcomes in NSCLC patients (9,31,34). For instance, compared to high aromatase expression alone, high expression of aromatase and ERβ predicted worse survival in both female and male patients, an effect that was more pronounced in women ≥65 years old (31).

Full table
Estrogen receptor and its impact on NSCLC prognostic
It is now recognized that NSCLC, particularly adenocarcinoma, is an estrogen receptor (ER) positive cancer. Estrogen receptor alpha (ERα) and beta (ERβ) have been identified in both NSCLC cell lines and tissues (27,35). ERβ is the predominant type of ER in NSCLC and is overexpressed in 60–80% of LC tissues. ERβ protein has been found at higher levels in lung tumors compared to normal lung tissue from the same patients, indicating its up-regulation in LC (35-37). Although ERβ expression has been observed in lung tumors from women and men (38), differences in its expression related to hormonal status have been reported. Recently, our group reported that tumors from premenopausal women have higher ERβ expression than tumors from postmenopausal women and men, suggesting that ERβ expression in LC is partially regulated by circulating estrogen (11). ERβ expression has also been shown to inversely correlate with the extent of tumor differentiation and with disease stage (31,39).
Further pointing to an important role of ERβ during lung carcinogenesis, several studies have shown that ERβ overexpression correlates with poor prognosis in LC patients (Table 1). Whereas ERβ expression in men is associated with good prognosis, in women it is associated with a worse prognosis (28,40,41). High expression of both aromatase and ERβ has been associated with poor survival predominantly in women >65 years old (31). In addition, a recent meta-analysis has shown that ERβ overexpression is significantly and consistently associated with poor survival outcomes in NSCLC patients (42).
Five ERβ isoforms have been identified (ERβ1 to 5) however ERβ-1 is the only fully functional isoform, capable of associating with its ligand (43). High cytoplasmic ERβ-1 staining has been identified as an independent negative prognostic factor for LC. In addition, the negative effect of ERβ-1 on survival has been observed in both male and female patients (9). ERβ-1 expression has been associated with more aggressive tumors and poor survival in metastatic LC, but not in early stage LC patients (44). Although the association between ER and poor prognoses in NSCLC is well documented, other studies have shown that ER-1 decreases the survival and induces apoptosis of NSCLC cells by regulating oncogenic RAS signaling and recently the involvement of ER-1 on NSCLC chemosensitivity to doxorubicin and etoposide has also been reported (45).
Future studies are needed to clarify the role of this ER isoform in lung carcinogenesis and the opposing function in the chemosensitivity to certain drugs.
Other isoforms of ERβ have been linked to better outcomes in LC but the mechanistic basis for this effect remains unknown (46).
On the other hand, ERα expression is less frequent in lung tumors (than that of ERβ) and it seems to be restricted to specific subsets of lung tumors such as those from patients with an EGFR mutation (47). In contrast to ERβ, ERα expression has been usually associated with good prognosis (48) particularly in patients with advanced stage disease (49). Staining of lung tumor tissues and cell lines have revealed that ERα is predominantly localized in the cytoplasm and on the cell membrane but rarely in the nucleus (50). Interestingly, while both ERβ and aromatase expression are correlated with worse prognosis and poor survival, expression of aromatase and ERα do not correlate with survival, suggesting that aromatase and ERβ, rather than ERα, are involved in lung carcinogenesis and tumor progression (40). Furthermore, microarray data have revealed that tumor expression of ERβ is associated with alterations in nearly 500 genes, (while ERα affected only 20 genes) which highlights the importance of ERβ in LC intracellular transformations (51)
Role of estrogen signaling in lung carcinogenesis
Recently there has been an increasing interest in establishing the underlying mechanisms by which estrogen promotes lung carcinogenesis. Upon activation with estrogen, ERs can activate signaling pathways leading to carcinogenesis through two main paths. ERs can translocate to the nucleus to regulate the transcription of genes (through the genomic pathway), or alternatively, they can translocate to the cell membrane to mediate the activation of protein kinases, second messengers and ion channels (through the non-genomic pathway) (Figure S1A).
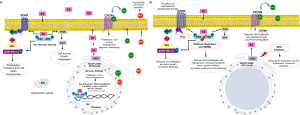
It has been shown that estrogen induces NSCLC cell proliferation and tumor growth while anti-estrogen drugs reduce tumor size and cell proliferation (30,50). Estrogen-induced cell proliferation results mainly from activation of cAMP, MAPK and AKT signaling pathways through the non-genomic pathway. Estradiol also promotes LC cell proliferation by inducing rapid phosphorylation of ERK and EGFR. Through the genomic pathway, estradiol promotes the expression of c-myc, Cyclin D, and Id proteins, resulting in cell cycle progression and proliferation (52,53). Furthermore, anti-apoptotic effects of estradiol have been reported in NSCLC as evidenced by studies of mitochondrial ERβ (54) (Figure S1A).
Angiogenesis plays a key role in cancer cell survival, localized tumor growth, and metastases and indeed estrogen signaling has been shown to induce tumor angiogenesis through vascular endothelial growth factor (VEGF) secretion, thereby increasing proliferation of vascular endothelial cells (55). Moreover, treatment with β-estradiol stimulates VEGFA expression, an important ligand for VEGFR-2 and the major VEGFR family member found in endothelial cells. Importantly, VEGFA secretion appears to be downstream of the estrogen-dependent activation of EGFR, which suggests that combining agents targeting EGFR and ER could synergistically inhibit this pathway. In agreement, it has been shown that the combination of fulvestrant and vandetanib successfully inhibited ER and EGFR crosstalk, which resulted in a greater reduction of proliferation in NSCLC as well as in the reduction in the angiogenic activity of the VEGFA-VEGFR2 pathway (56).
Recently, our group has reported the effects of estradiol on LC cell migration. We determined that estradiol stimulated CXCR4 expression in a dose- and a time-dependent manner and promoted the activation of the CXCL12/CXCR4 pathway, which increased the migration of LC cells in vitro. In contrast, treatment with the anti-estrogenic drug tamoxifen, decreased both the expression of CXCR4 and cell migration (57).
In addition, estrogen can promote NSCLC through G-protein-coupled estrogen receptor (GPER). Cytoplasmic and nuclear GPER signal has been detected in NSCLC samples and high expression of this protein has been associated with advanced stages of cancer, poor differentiation and high expression of ERβ. A recent report shows that G15 a selective inhibitor of GPER reverses the cell growth induced by E2, thus blocking of GPER signaling may be considered in the future as a new therapeutic target in NSCLC (58).
EGFR and ER pathway relationship in NSCLC
EGFR, a receptor tyrosine kinase, is overexpressed or mutated in up to 89% of NSCLC patients. Constitutive EGFR activation causes a reduction in cell death with a concomitant increase in cell survival, proliferation and migration, leading to increased tumorigenesis, angiogenesis, invasion and metastasis (59). Activated EGFR signaling also triggers enhanced expression of growth factors, particularly vascular endothelial growth factor (VEGF), fibroblast growth factor, platelet derived growth factor and interleukin-8 (60).
Interestingly, there is a relationship between the frequency of EGFR-activating mutations and patient demographics. EGFR mutations are more frequently found in patients from Peru (51.1%), East Asia (40%), Mexico (34.3%), as well as in tumors with adenocarcinoma histology (15–20%), in never-smokers (51%) and in women with NSCLC (42%) (61,62).
A functional relation between EGFR and ER signaling pathways was recently observed in lung adenocarcinoma (63,64). Stimulation of NSCLC with E2 resulted in a rapid activation of the EGFR pathway, suggesting a non-nuclear ER transactivation of EGFR (65) (Figure S1A). In addition, EGFR signaling activation increased the expression and activity of aromatase in NSCLC cells (30). Estrogen signaling induces epidermal growth factor (EGF) production, resulting in EGFR signaling activation (66).
Moreover, mutations in EGFR have been associated with ERβ expression; 67% of EGFR mutation positive tumors exhibit a high expression of nuclear ERβ versus 37% in EGFR wild-type tumors (67). Taken together, these data support the functional relationship between these two signaling pathways in NSCLC. Estrogen, through its receptor, activates downstream mediators of EGFR signaling such as MAPK and PI3K/AKT. Therefore, therapy based on anti-estrogen drugs might block downstream mediators from both ER and EGFR signaling pathways (68). The combined treatment with gefitinib and fulvestrant showed a synergic inhibitory effect on the proliferation and secretion of VEGF in NSCLC cells in vitro (69).
In addition, it has been reported that ERβ expression and EGFR mutations are prognostic factors for survival in patients with advanced NSCLC (70). Results from TORI L-03, a randomized multicenter phase II clinical trial using erlotinib plus fulvestrant, showed that patients with EGFR wild type tumors treated with this combination exhibited a higher clinical benefit rate than patients treated with erlotinib only (71). Furthermore, Collins and coworkers reported the clinical case of a female patient diagnosed with ER+, PR−, TTF-1+, EGFR+ stage IV adenocarcinoma treated with chemotherapy (based on cisplatin and vinorelbine) in combination with cetuximab and bisphosphonate. The patient showed a partial response and subsequently progressed. After 3 weeks of exemestane (aromatase inhibitor) treatment, the symptoms of the patient significantly improved, which corresponded with a 58% decrease in tumor size. This partial response was maintained for 6 months (72), suggesting that the exemestane-induced disruption of ER signaling affects EGFR signaling. Taken together these findings show that anti-hormonal therapy could be a new promising alternative for the treatment of LC.
Antiestrogenic therapy in NSCLC treatment
The evidence presented thus far, highlights the importance that aromatase, estrogen and ERs have in the development, progression and survival outcomes in LC. Consequently, the estrogen pathway represents an important source of actionable molecular targets for the development of new therapies for the treatment of LC patients. It has been suggested that blocking estrogen signaling, by inhibiting its synthesis, enhancing its degradation or blocking its putative receptors, could represent a novel and successful strategy in the treatment of several malignancies (Figure S1B). Indeed, such strategies are currently being developed and tested, particularly for NSCLC patients with adenocarcinoma histology, which as we have previously exposed is largely influenced by estrogen.
Levels of estrogen in the LC microenvironment are controlled in situ by the activity of aromatase, which produces 17-β-estradiol, and by estrogen sulfotransferase, which regulates estrogen metabolism by catalyzing the conversion of estrone to estrone 3-sulfate. These two enzymes have been the targets of antiestrogenic therapies in LC.
To date, there are ten clinical trials studying the effect and efficacy of cancer therapies targeting estrogen signaling, alone or in combination with other standard of care drugs as part of a multidrug-treatment therapy. Table 2 presents a comprehensive summary, showing a list of clinical trials targeting estrogen-signaling. Results from preclinical and clinical trials are summarized in Table 3.
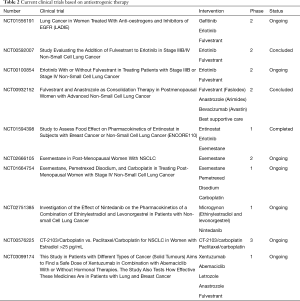
Full table
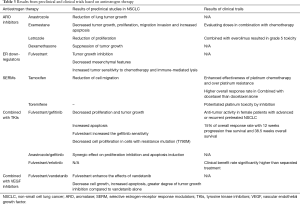
Full table
Aromatase inhibitors
Aromatase inhibitors are divided in two classes: (I) irreversible steroidal inhibitors, which form a strong bond with the aromatase and include drugs such as exemestane (Aromasin); (II) non-steroidal inhibitors, which reduce estrogen synthesis via reversible competition inhibition with the aromatase activity domain and include drugs such as anastrozole (Arimidex) and letrozole (Femara).
It has been suggested that patients with lung adenocarcinoma, mainly postmenopausal women with high aromatase expression, could be candidates for aromatase inhibitors. For instance, there is currently an ongoing phase I clinical trial evaluating the regimen limiting toxicity of exemestane (an aromatase inhibitor) in combination with chemotherapy for the treatment of postmenopausal women with advanced stage LC (NCT01664754). Additionally, treatment with exemestane showed suppressed NSCLC growth (30), decreased proliferation and increased apoptosis mainly in cells with higher aromatase levels (73). Other effects of exemestane include decreased cell migration and invasion, affecting mechanic cell properties (74). Similarly, treatment with anastrozole has been shown to reduce tumor growth both in in vivo and in vitro models of LC (26) and letrozole treatment reduces cell proliferation in ER positive LC cell lines, but a phase II study demonstrated that treatment with everolimus (mTOR inhibitor) combined with letrozole (aromatase inhibitor) in NSCLC caused grade 4 and 5 pulmonary toxicity, so combined treatment with letrozole must be approached with precaution (75).
Estrogen metabolism regulators
Genetic target activation of estrogen sulfotransferase has been successfully used in the treatment of hormonal dependent cancers. Activation of sulfotransferase by dexamethasone has been reported in A549 LC cells, resulting in decreased estrogen levels suppression of tumor growth. A recent preclinical study showed that dexamethasone, one of the most widely used synthetic glucocorticoids, exhibited promising anticancer properties in LC (76).
Estrogen receptor inhibitors
Fulvestrant is a selective estrogen receptor degrader (SERD), binds to the ER causing its destabilization and subsequent degradation. Recently, fulvestrant was tested in NSCLC preclinical studies. Treatment with estradiol promotes tumor growth, while treatment with fulvestrant (Faslodex) results in approximately 32% tumor growth inhibition in a mice model (65) and decreased ERβ expression (77).
Using NSCLC cells and a NSCLC xenograft model, it was demonstrated that co-treatment with fulvestrant and vandetanib (a multi-target inhibitor of VEGFR and EGFR) causes a synergic decrease in cell growth, an increase in apoptosis and a greater degree of tumor growth inhibition, indicating that fulvestrant enhances the anti-tumor effects of vandetanib in NSCLC through a mechanism that blocks estrogen-driven activation of the EGFR pathway (56). More recently, Hamilton and coworkers found that ERβ signaling correlates with both mesenchymal tumor features and with increased resistance to cell death inducers. In this study, it was shown that fulvestrant decreased mesenchymal features and increased tumor sensitivity to chemotherapy and immune-mediated lysis of LC cells (78).
Selective estrogen-receptor response modulators (SERMs)
Several studies have shown antitumor activity of SERMs, such as tamoxifen, raloxifene or toremifene, in both in vitro and in vivo assays. Recently, it was reported that estrogen treatment induced CXCR4 overexpression and increased cell migration trough the CXCR4/CXCL12 pathway. In contrast, tamoxifen treatment decreased CXCR4 expression and cell migration in NSCLC cell lines (57). Other preclinical studies have suggested that SERMs could enhance the effectiveness of platinum chemotherapy and overcome platinum resistance through PKC inhibition, which in turn downregulates c-FOS expression (79).
Furthermore, there is evidence showing that treatment of breast cancer patients with tamoxifen reduced the risk of developing additional primary LC tumors (80,81). Results from phase I and phase II clinical trials showed that tamoxifen and toremifene treatment potentiated platinum toxicity by PKC inhibition in NSCLC. High doses of toremifene and tamoxifen plus cisplatin had a good efficacy with safe and tolerable profiles in NSCLC patients previously treated with platinum compounds (82). In this study, the efficacy was better in the tamoxifen plus cisplatin arm (partial responses were observed in 4/10) (79). In contrast, in another study no significant differences were found with regards to response or survival rates (83).
Another recent trial evaluated the efficacy and safety of combined treatment with docetaxel and tamoxifen in advanced NSCLC patients who had received first line platinum-based chemotherapy. Results from this study showed that the overall response rate and disease control rate in the tamoxifen/docetaxel arm were significantly higher than those in the docetaxel group. Combined treatment, effectively reversed expression of P-glycoprotein-mediated-multidrug-resistance in tumors and provided a significant survival benefit in advanced NSCLC patients as compared to docetaxel treatment alone. Patients who exhibited reversed P-glycoprotein in the combined treatment group had a significantly better median progression-free survival and overall survival than non-reversal patients (84). In contrast, others studies have reported an opposite effect for SERMs, these compounds can act as both agonists and antagonists on ERs. The exact mechanism of action of SERMs in LC ERs is not yet clear, which warrants the need for an exhaustive characterization of the expression of ERs in normal tissue and LC. Thus further preclinical studies are necessary before SERMs can transition into clinical trials exploring their therapeutic potential in ER-expressing LC patients.
EGFR and ER targeting pathways
Antiestrogen therapy, combined with tyrosine kinase inhibitors (TKI) in EGFR positive lung tumors, has caused promising responses in patients with LC. Preclinical studies have shown that simultaneously targeting of ER and EGFR (1 µmol/L fulvestrant plus 1 µmol/L gefitinib) causes a 90% decrease in cell proliferation and a 2-fold increase in apoptosis. Furthermore, tumor growth was decreased by 60% in groups treated with the combination as compared to groups receiving single-agent treatment (65). Additionally, it has been shown that fulvestrant increases sensitivity to gefitinib in H1975 cells through the up-regulation of let-7c, which results in repression of RAS and inactivation of PI3K/AKT and MET/ERK signaling pathways (85). In another study, it was shown that inhibiting ER with anastrozole, and EGFR with gefitinib has a synergic effect on the inhibition of proliferation and induction of apoptosis of NSCLC cell lines as compared to the use of single-agent treatments (86).
Combined gefitinib and fulvestrant treatment has been applied to non-small LC cells with acquired resistance mutation to gefitinib (T790M). Combined treatment causes a significant decrease in the proliferation of T790 NSCLC cells, compared with separated treatments. A functional interaction between ERs and EGFR signaling pathways in a gefitinib-resistant cell line was observed (86). Thus, this type of combined therapy strategies could be used in lung tumors with acquired resistance mutations.
In a phase I clinical trial, gefitinib and fulvestrant co-treatment showed a good safety profile and significant anti-tumor activity in female patients with advanced or recurrent (stage IV) NSCLC, regardless of the prior lines of therapy they had received. In this study, 3/20 patients had partial responses with a 15% response rate, the median progression-free survival was 12 weeks and the median OS was 38.5 weeks. However, high ERβ expression in tumor specimens was associated with an overall survival of 65.5 weeks compared with 21 weeks in low ERβ expressing tumors, which indicates that patients with high ERβ expression would benefit the most, in terms of survival, from this combined treatment (87).
In a recent phase II trial, examining the efficacy of erlotinib and fulvestrant co-treatment vs. erlotinib treatment alone, it was found that among patients with EGFR wild-type tumors, the clinical benefit rate (which included partial responders and those with stable disease) was significantly higher among patients treated with both agents than among patients treated with erlotinib alone. Furthermore, in this study there was also a trend towards improved survival in the erlotinib-fulvestrant arm (88). Taken together, these studies support the notion that endocrine therapy, in combination with EGFR-TKI therapy, could be beneficial for both wildtype and EGFR mutation adenocarcinoma.
Estrogen influence on immune response in NSCLC
Cancer immunotherapy has recently emerged as the fifth and most successful strategy for the treatment of LC, along with surgery, radiation, chemotherapy and TKIs. The previous section explored the advantages of combined antiestrogen therapies with standard of care chemotherapeutic agents or TKIs. This section will highlight the importance and advantages of testing antiestrogen therapies in combination with immunotherapies. Perhaps the most compelling evidence for this is that steroid hormones are potent regulators of the immune system and key elements of the immune profile of patients, which together determine the efficacy of immunotherapeutic approaches for the treatment of LC.
It is now widely accepted that the immune system plays a key role in preventing or promoting the development and progression of several types of cancer, including LC, by a process known as “cancer immunoediting” (89). Similarly, it has been conclusively shown that males and females differ in their immunological responses (90,91). It is thus conceivable that cancer immunoediting is also different between males and females. Despite the burgeoning body of literature on the cellular and molecular mechanisms underlying each phase of the immunoediting process, very few studies analyze data by sex. Determining sex-based differences with regards to how the immune system interacts with the tumor, and vice versa, will form the basis for novel and truly personalized cancer immunotherapies.
Epidemiological studies have shown that adult females mount stronger innate and adaptive immune responses than males, which is a factor influencing their high susceptibility to develop inflammatory and autoimmune diseases. Interestingly, although chronic inflammation has been shown to initiate and promote cancer (92), females have a lower incidence and mortality rate for the majority of cancers than males.
EREs and androgen response elements (AREs) are found in the promoter regions of several innate immunity genes, indicating that sex hormones can differentially regulate innate immune responses. Estrogens also regulate adaptive immunity, as made evident by the fact that ERα and ERβ are up regulated in T-cells and B-cells, respectively (93). Furthermore, it has been shown that after chronic stimulation of male and female T-cells, the latter exhibit a higher degree of genetic up-regulation, particularly in immune response genes. Half of these up-regulated genes in female T-cells had EREs in their promoter regions.
It has been shown that that E2 treatment of female mice, previously infected with influenza A virus, increases the levels of CCL3 and CXCL1 (chemokines associated with neutrophil recruitment) as well as the frequency of lung infiltrating neutrophils. Similarly, a previous study showed that estrogen administration to healthy male volunteers causes a 2.3-fold increase in the frequency of circulating neutrophils. Taken together these studies provide evidence suggesting that estrogen levels can have a profound impact in the recruitment and the number of neutrophils, which is of particular relevance for LC studies, where neutrophilia is commonly reported. In the context of LC, neutrophils are recruited into the lung by the action of cytokines (94,95) where they exert a pro-tumorigenic effect (96,97). In NSCLC patients there is a strong correlation between poor clinical outcomes and high neutrophil content, both locally (98) and systemically (99-104). We have recently, reported that in addition to the known effects of IL-8 on neutrophil recruitment and expansion (95), CD47 overexpression on the surface of neutrophil causes a delay in their apoptosis and a reduction in their phagocytic clearance by macrophages, which may represent an important mechanism leading to neutrophilia in NSCLC patients (105). These findings highlight the importance of evaluating the clinical efficacy of antiestrogenic therapies in controlling neutrophil accumulation in NSCLC.
Interestingly, a new link has been found between estrogen and the activity of Natural Killer (NK) cells. NK cells exhibit natural cytotoxicity against a broad range of human solid tumors even in the absence of major histocompatibility complex molecules on the surface of target cells. Consequently, they play an important role in anti-tumor immunity (106). However, it is evident that clinically apparent tumors develop and progress even in the presence of NK cells, which indicates that malignant cells acquire mechanisms that allow them to evade or withstand the attack of NK cells (107). One such mechanism is the shedding of MICA and MICB, which impairs NKG2D function on NK cells, resulting in reduced antitumor responses. Interestingly, a recent study demonstrated that estradiol increased the mRNA levels of MICA and MICB as well as their secretion in NSCLC cells (A549 and LTEP-a2). This highlights once again the potential beneficial effects that antiestrogen drugs could have in stimulating innate anti-tumor immune responses.
Immunotherapies
Adverse effects to vaccination are more commonly reported among women than among men, which could reflect stronger immunogenic responses in females than in males or, alternatively, a case of reporting bias. In support of stronger immunogenic responses in women, it has been shown that following vaccination with inactivated influenza; females exhibit equivalent antibody titers to males with half the dose than that administered to males. These findings indirectly support the notion that immunogenicity to immunotherapies might also differ among the sexes. Although it is recognized that sex is a patient-associated factor of antibody immunogenicity, most clinical trials to date fail to report the rate and grade of adverse effects according to their occurrence among male and female patients.
There is a lack of information on potential sex-based differential responses (efficacy and dose-limiting toxicities) to anticancer immunotherapies (tumor targeting mAbs, peptide-based cancer vaccines, immunomodulatory mAbs). Antibody blockade of programmed death-1 (PD-1) or its ligand, PD-L1 has led to unprecedented therapeutic responses in NSCLC patients. Interestingly, there is preclinical evidence suggesting that antibodies against PD-L1 might elicit a stronger anti-tumor response in female patients than in male patients, due to a greater reduction in regulatory T cells (Treg) immunosuppressive function in females following anti-PD-L1 treatment. In this study it was shown that PD-L1−/− Tregs are highly sensitive to estrogen induction of their immunosuppressive activity (108), which indicates that responses to PD-L1 blocking therapies may differ between males and females, or between tumors with differential production of estrogen.
More recently it has been shown that estrogen promotes the progression of several estrogen-insensitive tumor models in vivo by impairing myelopoiesis and increasing the recruitment and immunosuppressive activity of myeloid derived suppressor cells (MDSCs) (109), which further supports the notion that combining antiestrogen drugs with immunotherapies may have a synergic effect in a variety of cancers, regardless of ER expression. These recent developments will surely bring with them the advent of a new plethora of cancer research trials, this time, evaluating the efficacy of antiestrogen drugs alone or in combination with immunotherapies.
Conclusions
A large and growing body of literature has accumulated highlighting the important role that estrogen and ERs have on the development and progression of LC. In particular, tumoral ER-β and aromatase expression have emerged not only as important prognostic factors associated with poor survival in NSCLC patients, but also as actionable molecular targets for the treatment of this malignancy. Anti-estrogenic drugs have been successfully used for the treatment of breast cancer; consequently, the information that is already available on these drugs (such as pharmacodynamics, pharmacokinetics, bioavailability, toxicities and dosing protocols alone or in combination with chemo/radiotherapy regimens) makes them ideal candidates to be repurposed for the treatment of LC patients. Despite the recent advancements on all fronts of thoracic oncology, the prognosis for patients with LC remains dismal. Indeed, only a small percentage of NSCLC patients are candidates to receive targeted therapies or immune checkpoint inhibitors, which offer a survival advantage. Antiestrogen therapy could be an additional therapeutic strategy that could result in better response rates in premenopausal women but also in male patients with ER+ and ARO+ lung tumors. Admittedly, there are still many areas of research on the role of estrogen and ERs that need to be explored in the context of lung carcinogenesis in order to identify the best combination of possible treatments. In particular, it is crucial that the exact molecular mechanisms by which estrogen and its receptors promote the development and progression of LC are elucidated. Finally, in the age of personalized medicine, it is essential that subsequent studies consider that there may be differences in the clinicopathological features, therapy response and survival of NSCLC patients that could be attributed to sex, and to the expression of hormonal markers. Nonetheless, there are several ongoing clinical trials evaluating the tolerability and efficacy of anti-estrogenic drugs alone or in combination with other standard of care agents for the treatment of NSCLC patients. The preliminary, updated data from some of these studies is encouraging and suggest that certain combinations do afford enhanced antitumor activity but confirmation of these findings is still awaited.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015;4:327-38. [PubMed]
- Vidyullatha P. Lung cancer incidence in never smokers: genetic and gender basis. Gene Rep 2016;4:19-207.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013;177:601-12. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Hsu LH, Liu KJ, Tsai MF, et al. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci 2015;106:51-9. [Crossref] [PubMed]
- Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011;17:154-64. [Crossref] [PubMed]
- Albain KS, Unger JM, Gotay CC, et al. Toxicity and survival by sex in patients with advance non-small cell lung carcinoma on modern Southwest Oncology Group (SWOG) trials. J Clin Oncol 2007;25:7549.
- Rodriguez-Lara V, Pena-Mirabal E, Baez-Saldana R, et al. Estrogen receptor beta and CXCR4/CXCL12 expression: differences by sex and hormonal status in lung adenocarcinoma. Arch Med Res 2014;45:158-69. [Crossref] [PubMed]
- Arrieta O, Quintana-Carrillo RH, Ahumada-Curiel G, et al. Medical care costs incurred by patients with smoking-related non-small cell lung cancer treated at the National Cancer Institute of Mexico. Tob Induc Dis 2015;12:25. [Crossref] [PubMed]
- Arrieta O, Campos-Parra AD, Zuloaga C, et al. Clinical and pathological characteristics, outcome and mutational profiles regarding non-small-cell lung cancer related to wood-smoke exposure. J Thorac Oncol 2012;7:1228-34. [Crossref] [PubMed]
- Tam A, Morrish D, Wadsworth S, et al. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 2011;11:24. [Crossref] [PubMed]
- Oton AB BC, Cai C, Owonikoko T, Gooding W, Siegfried J, et al. Comparison of survival for non-small cell lung cancer (NSCLC) between premenopausal and postmenopausal women: An analysis of the National Surveillance, Epidemiology and End Results (SEER) Database. J Clin Oncol 2006;24:7038.
- Ross H OF, Bandstra B, et al. Serum-free estradiol (E2) levels are prognostic in men with chemotherapy-naïve advanced non-small cell lung cancer and performance status (PS) 2. J Clin Oncol 2007;25:7683. (Meeting Abstr).
- Adami HO, Persson I, Hoover R, et al. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer 1989;44:833-9. [Crossref] [PubMed]
- Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol 2010;28:1540-6. [Crossref] [PubMed]
- Greiser CM, Greiser EM, Doren M. Menopausal hormone therapy and risk of lung cancer-Systematic review and meta-analysis. Maturitas 2010;65:198-204. [Crossref] [PubMed]
- Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst 2010;102:1413-21. [Crossref] [PubMed]
- Brinton LA, Schwartz L, Spitz MR, et al. Unopposed estrogen and estrogen plus progestin menopausal hormone therapy and lung cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Causes Control 2012;23:487-96. [Crossref] [PubMed]
- Bae JM, Kim EH. Hormonal replacement therapy and the risk of lung cancer in women: an adaptive meta-analysis of cohort studies. J Prev Med Public Health 2015;48:280-6. [Crossref] [PubMed]
- Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243-51. [Crossref] [PubMed]
- Chlebowski RT, Wakelee H, Pettinger M, et al. Estrogen Plus Progestin and Lung Cancer: Follow-up of the Women's Health Initiative Randomized Trial. Clin Lung Cancer 2016;17:10-7.e1. [Crossref] [PubMed]
- Ganti AK, Sahmoun AE, Panwalkar AW, et al. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol 2006;24:59-63. [Crossref] [PubMed]
- Weinberg OK, Marquez-Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res 2005;65:11287-91. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Skjefstad K, Grindstad T, Khanehkenari MR, et al. Prognostic relevance of estrogen receptor alpha, beta and aromatase expression in non-small cell lung cancer. Steroids 2016;113:5-13. [Crossref] [PubMed]
- Abe K, Miki Y, Ono K, et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol 2010;41:190-8. [Crossref] [PubMed]
- Márquez-Garbán DC, Chen HW, Goodglick L, et al. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci 2009;1155:194-205. [Crossref] [PubMed]
- Mah V, Marquez D, Alavi M, et al. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer 2011;74:318-25. [Crossref] [PubMed]
- Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res 2007;67:10484-90. [Crossref] [PubMed]
- Tanaka K, Shimizu K, Kakegawa S, et al. Prognostic significance of aromatase and estrogen receptor beta expression in EGFR wild-type lung adenocarcinoma. Am J Transl Res 2016;8:81-97. [PubMed]
- Aresti U, Carrera S, Iruarrizaga E, et al. Estrogen receptor 1 gene expression and its combination with estrogen receptor 2 or aromatase expression predicts survival in non-small cell lung cancer. PLoS One 2014;9:e109659. [Crossref] [PubMed]
- Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol 2009;36:524-31. [Crossref] [PubMed]
- Chakraborty S, Ganti AK, Marr A, et al. Lung cancer in women: role of estrogens. Expert Rev Respir Med 2010;4:509-18. [Crossref] [PubMed]
- Siegfried JM. Smoking out reproductive hormone actions in lung cancer. Mol Cancer Res 2014;12:24-31. [Crossref] [PubMed]
- Olivo-Marston SE, Mechanic LE, Mollerup S, et al. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis 2010;31:1778-86. [Crossref] [PubMed]
- Chen XQ, Zheng LX, Li ZY, et al. Clinicopathological significance of oestrogen receptor expression in non-small cell lung cancer. J Int Med Res 2017;45:51-8. [Crossref] [PubMed]
- Baik CS, Eaton KD. Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel) 2012;4:969-88. [Crossref] [PubMed]
- Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280-7. [Crossref] [PubMed]
- Ma L, Zhan P, Liu Y, et al. Prognostic value of the expression of estrogen receptor beta in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2016;5:202-7. [Crossref] [PubMed]
- Leung YK, Mak P, Hassan S, et al. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci U S A 2006;103:13162-7. [Crossref] [PubMed]
- Navaratnam S, Skliris G, Qing G, et al. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm Cancer 2012;3:93-100. [Crossref] [PubMed]
- Nikolos F, Thomas C, Bado I, et al. ERbeta Sensitizes NSCLC to Chemotherapy by Regulating DNA Damage Response. Mol Cancer Res 2017. [Crossref] [PubMed]
- Liu Z, Liao Y, Tang H, et al. The expression of estrogen receptors beta2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine 2013;44:517-24. [Crossref] [PubMed]
- Mazière J, Rouquette I, Lepage B, et al. Specificities of lung adenocarcinoma in women who have never smoked. J Thorac Oncol 2013;8:923-9. [Crossref] [PubMed]
- Berardi R, Morgese F, Santinelli A, et al. Hormonal receptors in lung adenocarcinoma: expression and difference in outcome by sex. Oncotarget 2016;7:82648-57. [Crossref] [PubMed]
- Li W, Tse LA, Wang F. Prognostic value of estrogen receptors mRNA expression in non-small cell lung cancer: A systematic review and meta-analysis. Steroids 2015;104:129-36. [Crossref] [PubMed]
- Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 2002;62:2141-50. [PubMed]
- Kerr A 2nd, Eliason JF, Wittliff JL. Steroid receptor and growth factor receptor expression in human nonsmall cell lung cancers using cells procured by laser-capture microdissection. Adv Exp Med Biol 2008;617:377-84. [Crossref] [PubMed]
- Hershberger PA, Vasquez AC, Kanterewicz B, et al. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res 2005;65:1598-605. [Crossref] [PubMed]
- Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol 2014;41:5-16. [Crossref] [PubMed]
- Zhang G, Yanamala N, Lathrop KL, et al. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol Endocrinol 2010;24:1737-47. [Crossref] [PubMed]
- Marquez-Garban DC, Mah V, Alavi M, et al. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 2011;76:910-20. [PubMed]
- Siegfried JM, Gubish CT, Rothstein ME, et al. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol 2012;7:485-95. [Crossref] [PubMed]
- Rodriguez-Lara V, Ignacio GS, Cerbon Cervantes MA. Estrogen induces CXCR4 overexpression and CXCR4/CXL12 pathway activation in lung adenocarcinoma cells in vitro. Endocr Res 2017;42:219-31. [PubMed]
- Liu C, Liao Y, Fan S, et al. G-protein-coupled estrogen receptor antagonist G15 decreases estrogen-induced development of non-small cell lung cancer. Oncol Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bethune G, Bethune D, Ridgway N, et al. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis 2010;2:48-51. [PubMed]
- Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res 2000;60:2926-35. [PubMed]
- Prabhakar CN. Epidermal growth factor receptor in non-small cell lung cancer. Transl Lung Cancer Res 2015;4:110-8. [PubMed]
- Arrieta O, Cardona AF, Martin C, et al. Updated Frequency of EGFR and KRAS mutations in non-small-cell lung cancer in Latin America: The Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J Thorac Oncol 2015;10:838-43. [Crossref] [PubMed]
- Giovannini M, Belli C, Villa E, et al. Estrogen receptor (ER) and epidermal growth factor receptor (EGFR) as targets for dual lung cancer therapy: not just a case? J Thorac Oncol 2008;3:684-5. [Crossref] [PubMed]
- Ling YH, Li T, Perez-Soler R, et al. Activation of ER stress and inhibition of EGFR N-glycosylation by tunicamycin enhances susceptibility of human non-small cell lung cancer cells to erlotinib. Cancer Chemother Pharmacol 2009;64:539-48. [Crossref] [PubMed]
- Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005;65:1459-70. [Crossref] [PubMed]
- Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 2003;17:309-17. [Crossref] [PubMed]
- Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 2009;27:411-7. [Crossref] [PubMed]
- Kawai H. Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J Clin Oncol 2014;5:1020-7. [Crossref] [PubMed]
- Pietras RJ, Marquez DC, Chen HW, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 2005;70:372-81. [Crossref] [PubMed]
- Deng F, Li M, Shan WL, et al. Correlation between epidermal growth factor receptor mutations and the expression of estrogen receptor-beta in advanced non-small cell lung cancer. Oncol Lett 2017;13:2359-65. [Crossref] [PubMed]
- Garon EB, Pietras RJ, Finn RS, et al. Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer. J Thorac Oncol 2013;8:270-8. [Crossref] [PubMed]
- Collins IM, Nicholson SA, O'Byrne KJ. A lung cancer responding to hormonal therapy. J Thorac Oncol 2010;5:749-50. [Crossref] [PubMed]
- Koutras A, Giannopoulou E, Kritikou I, et al. Antiproliferative effect of exemestane in lung cancer cells. Mol Cancer 2009;8:109. [Crossref] [PubMed]
- Giannopoulou E, Siatis KE, Metsiou D, et al. The inhibition of aromatase alters the mechanical and rheological properties of non-small-cell lung cancer cell lines affecting cell migration. Biochim Biophys Acta 2015;1853:328-37. [Crossref] [PubMed]
- Singhal N, Vatandoust S, Brown MP. Phase II study evaluating efficacy and safety of everolimus with letrozole for management of advanced (unresectable or metastatic) non-small cell lung cancer after failure of platinum-based treatment: a preliminary analysis of toxicity. Cancer Chemother Pharmacol 2015;75:325-31. [Crossref] [PubMed]
- Wang LJ, Li J, Hao FR, et al. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta Pharmacol Sin 2016;37:845-56. [Crossref] [PubMed]
- Tang H, Liao Y, Zhang C, et al. Fulvestrant-mediated inhibition of estrogen receptor signaling slows lung cancer progression. Oncol Res 2014;22:13-20. [Crossref] [PubMed]
- Hamilton DH, Griner LM, Keller JM, et al. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res 2016;22:6204-16. [Crossref] [PubMed]
- Perez EA, Gandara DR, Edelman MJ, et al. Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer Invest 2003;21:1-6. [Crossref] [PubMed]
- Chu SC, Hsieh CJ, Wang TF, et al. Antiestrogen use in breast cancer patients reduces the risk of subsequent lung cancer: A population-based study. Cancer Epidemiol 2017;48:22-8. [Crossref] [PubMed]
- Rosell J, Nordenskjold B, Bengtsson NO, et al. Long-term effects on the incidence of second primary cancers in a randomized trial of two and five years of adjuvant tamoxifen. Acta Oncol 2017;56:614-7. [Crossref] [PubMed]
- Lara PN Jr, Gandara DR, Longmate J, et al. Activity of high-dose toremifene plus cisplatin in platinum-treated non-small-cell lung cancer: a phase II California Cancer Consortium Trial. Cancer Chemother Pharmacol 2001;48:22-8. [Crossref] [PubMed]
- Cheng H, Wu Y, Gu L, et al. The preliminary results of a phase II randomized clinical trial of high-dose toremifene chemosensitization in stage IIIB/IV non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2003;6:335-8. [PubMed]
- Wen S, Fu X, Li G, et al. Efficacy of tamoxifen in combination with docetaxel in patients with advanced non-small-cell lung cancer pretreated with platinum-based chemotherapy. Anticancer Drugs 2016;27:447-56. [Crossref] [PubMed]
- Shen H, Yuan Y, Sun J, et al. Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomed Pharmacother 2010;64:88-92. [Crossref] [PubMed]
- Xu R, Shen H, Guo R, et al. Combine therapy of gefitinib and fulvestrant enhances antitumor effects on NSCLC cell lines with acquired resistance to gefitinib. Biomed Pharmacother 2012;66:384-9. [Crossref] [PubMed]
- Traynor AM, Schiller JH, Stabile LP, et al. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer 2009;64:51-9. [Crossref] [PubMed]
- Garon ES, Siegfried JM, Dubinett SM, et al, editor. Results of TORI-L-03, a randomized, multicenter phase II clinical trial of erlotinib (E) or E + fulvestrant (F) in previously treated advanced nonsmall cell lung cancer (NSCLC). Proceedings of the 104th Annual Meeting of the American; 2013; Washington DC. Philadelphia.
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626-38. [Crossref] [PubMed]
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 2012;11:A479-85. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 2015;294:63-9. [Crossref] [PubMed]
- Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol 1998;152:83-92. [PubMed]
- De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004;10:4895-900. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [Crossref] [PubMed]
- Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 2012;118:1726-37. [Crossref] [PubMed]
- Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015;5:12493. [Crossref] [PubMed]
- Peng B, Wang YH, Liu YM, et al. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med 2015;8:3098-106. [PubMed]
- Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524-30. [Crossref] [PubMed]
- Arrieta O, Michel Ortega RM, Villanueva-Rodriguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [Crossref] [PubMed]
- Carus A, Gurney H, Gebski V, et al. Impact of baseline and nadir neutrophil index in non-small cell lung cancer and ovarian cancer patients: Assessment of chemotherapy for resolution of unfavourable neutrophilia. J Transl Med 2013;11:189. [Crossref] [PubMed]
- Sanchez-Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer 2012;64:526-34. [Crossref] [PubMed]
- Barrera L, Montes-Servin E, Hernandez-Martinez JM, et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 2017;117:385-97. [Crossref] [PubMed]
- Bryceson YT, March ME, Ljunggren HG, et al. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006;107:159-66. [Crossref] [PubMed]
- Screpanti V, Wallin RP, Grandien A, et al. Impact of FASL-induced apoptosis in the elimination of tumor cells by NK cells. Mol Immunol 2005;42:495-9. [Crossref] [PubMed]
- Lin PY, Sun L, Thibodeaux SR, et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol 2010;185:2747-53. [Crossref] [PubMed]
- Svoronos N, Perales-Puchalt A, Allegrezza MJ, et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov 2017;7:72-85. [Crossref] [PubMed]


