Assessment of the mitral valve coaptation zone with 2D and 3D transesophageal echocardiography before and after mitral valve repair
Introduction
Mitral valve (MV) repair has significant advantages over valve replacement for treating mitral regurgitation (MR) (1-3). The ultimate objective of surgical valve repair is to restore the largest possible leaflet coaptation surface (1,4-6). Several investigators have assessed the changes in the geometry of the MV after valve repair, using ultrasound or magnetic resonance imaging. Information about the mitral coaptation zone, especially the degree of MV coaptation, has been evaluated in some clinical settings (7-9). It has been suggested that valvular regurgitation can be predicted through accurate quantification of the degree of MV coaptation.
The coaptation length index (CLI), which is defined as the ratio of the coaptation length to the anterior MV leaflet length, is a two-dimensional transesophageal echocardiography (2D TEE) index (7,10). The traditional 2D TEE method can be used to measure the regional CLI of coaptation in patients with MR and to assess the interaction between the degree of mitral coaptation and MR severity. However, the 2D TEE method is fundamentally limited to one-dimensional measurement.
Three-dimensional transesophageal echocardiography (3D TEE) using the 3D planimetric method provides excellent images of the MV geometry and accurately measures the mitral coaptation zone (11,12). The coaptation area index (CAI), which is defined as the ratio of the MV coaptation area to the leaflet area, is a 3D TEE index (8,12,13). However, due to its limited accessibility and financial constraints, 2D TEE methods of measuring MV coaptation that are as efficient as 3D planimetry should be explored.
This study extends the traditional 2D TEE method to analyze multiple zones of coaptation simultaneously, using 3D TEE. We quantified and compared the 2D TEE measurement of the CLI and 3D TEE measurement of the CAI in patients undergoing MV repair. We also aimed to elucidate whether the 2D TEE method is as efficient as the 3D TEE planimetric method to assess MV coaptation.
Methods
Patients
Between May 2014 and June 2017, 48 patients who underwent MV repair for nonrheumatic MR were selected as study subjects. The Carpentier functional classification of valve lesions was type II (leaflet prolapse). All patients undergoing cardiac surgery underwent 2D TEE and 3D TEE, which were recorded as part of a routine intraoperative echocardiographic protocol. A preoperative Doppler-echo analysis showed the presence of moderate to severe mitral insufficiency (grade 3/4) or severe regurgitation (grade 4/4), evaluated using a scale from 0 to 4, in all patients.
The inclusion criteria were: (I) MR due to mitral leaflet prolapse (Carpentier type II); (II) normal left ventricular function with a left ventricle ejection fraction (LVEF) greater than 50%; and (III) an MR grade greater than moderate (grade 3/4–4/4).
The exclusion criteria were: (I) subvalvular lesions (e.g., those seen in rheumatic disease); (II) another cardiac disease (e.g., pericardial, ischemic, congenital, or infiltrative heart disease); and (III) atrial fibrillation.
The study was approved by the ethics committee of our hospital (No. 2017024X), and written consent was obtained from all subjects.
Operative procedure
Patients underwent MV repair using several techniques on the mitral leaflets and chordae tendineae including ring annuloplasty, edge-to-edge repair (ETER), chordal shortening and transfer, leaflet resection, and artificial chordae. Ring annuloplasty was performed in all cases, ETER in 22 cases, chordal shortening and transfer in 28 cases, partial leaflet resection in 10 cases, and artificial chordae were added in 10 cases. The implanted rings were Carpentier-Edwards Physio Annuloplasty Rings (Edwards Lifesciences, Irvine, CA, USA).
Echocardiographic protocol
All of the echocardiographic examinations were performed using ultrasound equipment (IE33 system; Philips Medical Systems, Andover, MA, USA), with an X7-2 probe for both 2D and 3D images. In the intraoperative pre- and post-cardiopulmonary bypass periods, complete 2D TEE and 3D TEE examinations were performed. All the echocardiographic parameters were measured in both the preoperative and postoperative periods. The severity of MR was graded on a 0 to 4 scale (0=none, 1=mild, 2=moderate, 3=moderate to severe, and 4=severe), based on how far regurgitation extended into the left atrium beyond the MV. The LVEF was measured using the biplane Simpson’s method (14,15).
Quantification of MV coaptation with 2D-TEE
The MV consists of three pairs of corresponding segments: lateral (A1/P1), middle (A2/P2), and medial (A3/P3). The MV was examined in multiple cross-sectional views. The MV was imaged in mid-esophageal (ME) long-axis views at 120° to 150°, which allowed us to acquire views of the lateral, middle, and medial segments (A1/P1, A2/P2, and A3/P3). The entire length of the anterior mitral leaflet (AML) during the diastolic phase (Ad) and the length of the uncoapted AML in the end-systole phase (Ac) were measured at the A1/P1, A2/P2, and A3/P3 sites (Figure 1).
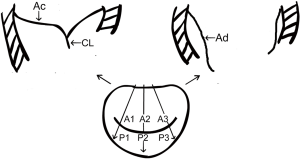
The coaptation length (CL) was defined according to the following equation:
CL = Ad − Ac
The CLI was defined as and could be calculated from the following equation:
CLI = CL/Ad ×100%
The CL and CLI were measured at A1/P1, A2/P2, and A3/P3. The average values of the three regions were measured.
Quantification of MV coaptation using 3D TEE
3D images of the MV were acquired using a real-time 3D zoom mode in a single cardiac cycle. The volumetric frame rate (live 3D zoom mode) was 10 Hz. To evaluate the degree of leaflet coaptation, we manually traced the leaflets in the following two frames: (I) at the onset of MV closure; and (II) at the maximum MV closure. The total leaflet area was assessed at the onset of mitral leaflet closure. The changes in the tenting surface area in these two frames reflected the degree of MV coaptation (7,11-13,16) (Figure 2).
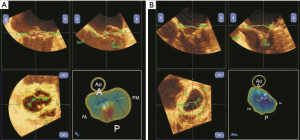
The coaptation area (CA) was defined according to the following equation:
CA = total leaflet area (TLA) − closed leaflet area in mid-systole (CLA)
The CAI was calculated using the following equation:
CAI = CA/TLA ×100%
All echocardiographic examinations were performed by the same operator. To calculate the intraobserver variability, we re-measured the stored data of 10 randomly chosen examinations one week later (1-week interval).
Statistical analysis
The statistical analysis was performed using the PASW Statistics for Windows, Version 18.0 software (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. Comparisons between different groups were assessed using the chi-square and t-tests. Spearman’s rank correlation analysis was applied to analyze the relationship between the degree of MR and each parameter. Furthermore, Pearson's correlation analysis was used to assess the correlation between the CLI and CAI, including both preoperative and postoperative findings. Reproducibility was assessed using the intraclass correlation coefficient. Significance was assessed at a P of less than 0.05.
Results
Patient characteristics
The study enrolled 26 (54.17%) male and 22 (45.83%) female subjects with an average age of 52.23±13.31 years. The patients’ characteristics, including age, body surface area, and clinical comorbidities are shown in Table 1. The causes of valve insufficiency were degenerative MV disease or a ruptured chordae tendineae, including fibroelastic deficiency in 45 patients (93.75%), Barlow disease in 1 patient (2.08%), and endocarditis in 2 patients (4.17%).

Full table
In the 48 cases studied, P2 prolapse was found in 11 patients (including 8 patients with P2 flail), P3 prolapse in 10 patients (including 5 patients with P2 flail), A2 prolapse in 14 patients (including 2 patients with A2 flail), A2P2 prolapse in 9 patients (including 2 patients with P2 flail), and A3P3 prolapse in 4 patients.
Ring implantation was attempted and successful in all patients. The implanted ring sizes were 28 mm (n=11), 30 mm (n=16), 32 mm (n=16), and 34 mm (n=5). Furthermore, the average annular size was 30.67±1.90 mm. In addition, ETER occurred in 22 cases (45.83%), chordal shortening and transfer in 28 cases (58.33%), partial leaflet resection in 10 cases (20.83%), and artificial chordae were added in 10 cases (20.83%). Preoperatively, the MR grade was 3/4 in 5 patients (10.41%) and 4/4 in 43 patients (89.58%). Postoperatively, the residual MR grade was 0/4 in 12 patients (25%) and 1/4 in 36 patients (75%).
Comparison of the 2D and 3D TEE measurements in the assessment of MV coaptation
The CL and CLI were successfully measured using 2D TEE in 46 (95.83%) subjects, and the CA and CAI were successfully measured using 3D TEE in 39 (81.25%). The success rate of the 2D TEE measurement method was significantly higher that of 3D TEE (χ2=5.03; P=0.03).
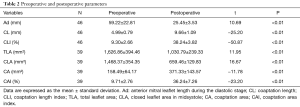
The changes in each echocardiographic parameter from preoperatively to postoperatively are summarized in Table 2. After MV surgery, the coaptation indexes, including the Ad, TLA, and CLA, were significantly decreased after adjustment than before adjustment, whereas the CL, CLI, CA, and CAI were significantly increased.
Full table
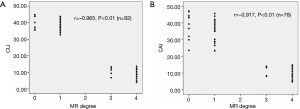
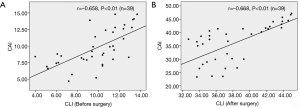
Spearman’s rank-order correction analysis showed that there was a significant and negative correlation between the severity grade of MR and 2D CLI (r=−0.97, P<0.01) or 3D CAI (r=−0.92, P<0.01) (Figure 3). Furthermore, Pearson's correlation analysis revealed that 2D CLI significantly correlated with 3D CAI (preoperatively: r=0.66, P<0.01; postoperatively: r=0.67, P<0.01) (Figure 4).
The intraobserver agreement for the measurements of the CLI and CAI were high, with an intraclass correlation coefficient of 0.95 for the CLI and 0.92 for the CAI.
Discussion
MV repair is an effective surgical strategy that has significant advantages over valve replacement for treating patients with MR (1-3). One of the primary goals of MV repair is that a large area of leaflet coaptation can be created (1,4-6). The degree of MV leaflet coaptation should be an important parameter when assessing the valve geometry and function pre- and postoperatively. Two methods have been used to evaluate the actual degree of the MV coaptation zone in the clinical setting, including 2D TEE and 2D TEE (7,9).
The traditional 2D TEE method is a simplified method that simply and directly measures the length of mitral leaflet coaptation, but 2D TEE is fundamentally limited to one-dimensional measurement. Spatial orientation maybe difficult to determine for 2D TEE, and the interpretation of 2D TEE images often requires a high level of expertise. The 3D TEE method is a novel technology that provides excellent images of the MV geometry and accurate measurements of the mitral coaptation zone. Despite the advantages of 3D TEE, it has issues such as limited accessibility and financial constraints still exist. The analysis of 3D TEE data requires specific software and is time-consuming, which limit its widespread clinical availability. Therefore, this raises the question as to whether the conventional 2D TEE method is likely to be as efficient as the 3D TEE method. However, there is a paucity of information in the literature on this topic. Moreover, the relationship between the 2D TEE and 3D TEE methods remains unclear.
Our 2D TEE method could allow us to determine the CL by subtracting the length of the uncoapted anterior leaflet in the end-systole phase from the whole length of the anterior leaflet that is measured during the diastolic stage, based on the finding that the length of the anterior leaflet is unchanged during the cardiac cycle on 2D TEE images. This may be mainly because of the difficulty in measuring the exact CL in clinical settings and partially because the exact point of leaflet coaptation is usually unclear. Furthermore, occasionally, the CL is too short to directly measure it (7,17).
In our study, the Ad, CL, and CLI were used as 2D TEE indexes, and TLA, CLA, CA, and CAI were 3D TEE indexes. After MV surgery, the coaptation indexes, including Ad, TLA, and CLA, were significantly decreased compared with those before surgery, whereas the CL, CLI, CA, and CAI were significantly increased. Thus, MV repair improved mitral leaflet coaptation in patients with MR due to an increase in the CL, CLI, CA, and CAI. We found that the CLI and CAI were associated with the severity of MR, both before and after surgery. These results highlight the importance of the CLI and CAI in determining the degree of MR. More importantly, we also found that the CLI was significantly correlated with the CAI. These findings suggest that CLI measurements that are obtained using the 2D TEE method have a similar value to CAI measurements that are obtained using the 3D-TEE method. In the present study, 27 of 48 (56.25%) patients with anterior leaflet prolapse, and 22 of 27 (81.48%) anterior leaflet prolapse cases were primarily repaired by ETER. This may help to explain why there was a more than half size reduction in the anterior leaflet length postoperatively.
This study revealed the low feasibility of 3D TEE compared with 2D-TEE (39 vs. 48 Hz). The causes of the lower feasibility of 3D-TEE when compared to 2D-TEE is that some patients with severe MR had an exceedingly large mitral annular dimension. When using the 3D zoom mode acquisition, it was difficult to cover the whole MV in those patients. Therefore, 9 of the 48 patients who were screened were excluded from the 3D TEE study for this reason. The 2D TEE provides images with a higher frame rate (49 Hz) than the live 3D zoom mode (10 Hz) currently available in the clinical setting, which is one of the advantages of 2D-TEE.
The study’s limitations are as follows. First, the study only focused on MR patients with leaflet prolapse. Not all pathologies of MR were included, and the applicability of this study in patients with other MR pathologies (e.g., ischemic or rheumatic disease) needs to be assessed in future studies. Second, there are various valve repair techniques, including classical resection, neochordae, and edge-to-edge repair. Due to the small sample size of subgroups for valve repair techniques, the effect of different operative procedures on our study results needs to be evaluated further.
In summary, we compared two different ultrasound technologies to assess the results of MV repair, and found that the degree of MV coaptation significantly increased after MV repair. The 2D TEE CLI and 3D TEE CAI are closely associated with the severity of MR before and after surgical repair. The 3D TEE CAI parameter is more quantitative and accurate than the 2D TEE CLI parameter, but the 2D TEE CLI parameter is simple and does not take much time. In addition, the coaptation indexes that are calculated using 2D TEE can provide a similar value to that of the 3D TEE method. Therefore, the 2D TEE CLI can be applied as an alternative to the 3D TEE CAI when assessing MV coaptation, due to its simplicity.
Acknowledgements
Funding: This work was supported by Cultivation of high level health technical personnel in Beijing health system (No. 2015-3-409).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of our hospital (No. 2017024X), and written consent was obtained from all subjects.
References
- Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998;116:734-43. [Crossref] [PubMed]
- Maisano F, La Canna G, Grimaldi A, et al. Annular-to-leaflet mismatch and the need for reductive annuloplasty in patients undergoing mitral repair for chronic mitral regurgitation due to mitral valve prolapse. Am J Cardiol 2007;99:1434-9. [Crossref] [PubMed]
- Gillinov AM, Cosgrove DM 3rd, Shiota T, et al. Cosgrove-Edwards annuloplasty system: Midterm results. Ann Thorac Surg 2000;69:717-21. [Crossref] [PubMed]
- Bax JJ, Braun J, Somer ST, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 2004;110:II103-8. [Crossref] [PubMed]
- Greenhouse DG, Dellis SL, Schwartz CF, et al. Regional changes in coaptation geometry after reduction annuloplasty for functional mitral regurgitation. Ann Thorac Surg 2012;93:1876-80. [Crossref] [PubMed]
- Padala M, Powell SN, Croft LR, et al. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J Thorac Cardiovasc Surg 2009;138:309-15. [Crossref] [PubMed]
- Yamauchi T, Taniguchi K, Kuki S, et al. Evaluation of the mitral valve leaflet morphology after mitral valve reconstruction with a concept “coaptation length index.” J Card Surg 2005;20:432-5. [Crossref] [PubMed]
- Yamada R, Watanabe N, Kume T, et al. Quantitative measurement of mitral valve coaptation in functional mitral regurgitation: in vivo experimental study by real time three-dimensional echocardiography. J Cardiol 2009;53:94-101. [Crossref] [PubMed]
- Cobey FC, Swaminathan M, Phillips-Bute B, et al. Quantitative assessment of mitral valve coaptation using three-dimensional transesophageal echocardiography. Ann Thorac Surg 2014;97:1998-2004. [Crossref] [PubMed]
- Shudo Y, Matsue H, Toda K, et al. A simplified echocardiographic measurements of direct effects of restrictive annuloplasty on mitral valve geometry. Echocardiography 2010;27:931-6. [Crossref] [PubMed]
- Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol 2008;52:446-9. [Crossref] [PubMed]
- Gogoladze G, Dellis SL, Donnino R, et al. Analysis of the mitral coaptation zone in normal and functional regurgitant valves. Ann Thorac Surg 2010;89:1158-61. [Crossref] [PubMed]
- Saito K, Okura H, Watanabe N, et al. Influence of chronic tethering of the mitral valve on mitral leaflet size and coaptation in functional mitral regurgitation. JACC Cardiovasc Imaging 2012;5:337-45. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echcardiography, a branch of the European Socety of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [Crossref] [PubMed]
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358-67. [Crossref] [PubMed]
- Chen L, May-Newman K. Effect of strut chordae transection on mitral valve leaflet biomechanics. Ann Biomed Eng 2006;34:917-26. [Crossref] [PubMed]
- Calafiore AM, Gallina S, Di Mauro M, et al. Mitral valve procedure in dilated cardiomyopathy: Repair or replacement? Ann Thorac Surg 2001;71:1146-52; discussion 1152-3. [Crossref]


