PTEN gene silencing contributes to airway remodeling and induces airway smooth muscle cell proliferation in mice with allergic asthma
Introduction
Allergic asthma is a chronic airway inflammatory disease associated with a series of aberrant immune responses, which is triggered due to exposure of genetically susceptible individuals to environmental stimuli (1). Allergic asthma has been termed as a “syndrome” resulting from a complex interplay between genetic and environmental factors (2). It has been reported that more than 300 million people worldwide suffer from asthma and this figure is predicted to increase by another 100 million by 2025 (3). A study highlighted that the prevalence of asthma increased remarkably in the past two decades, especially in children (4). Allergic asthma has been found to be induced by common allergens, such as inhalants, foods, pollens, drugs, house dust, occupationally encountered dust and animal dander (5). Airway remodeling is an important feature of asthma, and is characterized by an increase in the number of airway smooth muscle cells (ASMCs) in the airway wall (6). Airway remodeling refers to the structural changes including loss of epithelial integrity (7), thickening of the basement membrane (8), sub-epithelial fibrosis (9), goblet cell and submucosal gland enlargement (10), increased smooth muscle mass and increased airway vascularity (11). In modern medicine, gene-based therapies for cancer been wildly adopted in clinical trials and include strategies involving chemotherapeutic approaches and augmentation of immune-therapeutics (12).
Phosphatase and tensin homolog (PTEN) gene is located on chromosome 10q23 and it shares extensive homology with cytoskeletal proteins auxilin and tensin (13). PTEN is a tumor suppressor that gets mutated along the development of human cancer like gastric cancer (14). In the recent years, participation of PTEN gene in asthma pathogenesis has been observed, where its low expression is considered as one of the independent factors in the development of asthma (15). It primarily functions as a lipid phosphatase for the regulation of crucial signal transduction pathways (16). Loss of PTEN gene function followed by activation of PI3K/Akt signaling pathway has been associated with tumor occurrence (17). PTEN gene silencing has been reported to promote cancer progression in ways, such as increasing genomic instability, stem cell self-renewal, and the acceleration of cellular senescence and metastasis (18). It was gradually recognized that airway smooth muscle (ASM) could produce several airway-disease mediators (e.g., growth factors and cytokines) that could have both positive and negative effects on airway narrowing and remodeling (19). Since PTEN involvement in many types of cancer progressions has been observed, thereby we would like to investigate its role in the proliferation of ASMCs. Therefore, our study aims to use gene silencing in order to explore the effects of PTEN gene silencing on ASMCs proliferation and airway remodeling in allergic asthmatic mouse.
Methods
Ethics statement
All mice were treated in strict accordance with the national animal experiment rules (18). The study was performed with approval of the animal ethics association of the Affiliated Municipal Hospital of Xuzhou (permit number: 201603002), and all efforts were made to minimize animal suffering.
Preparation of si-PTEN plasmid
The specific siRNA of PTEN was designed and synthesized by Qiagen Company (Hilden, Germany). Its silence efficiency was tested and confirmed to be over 80%, and the effective duration of silence was between 4 to 10 days.
Study subjects
A total of 56 healthy female BABL/c mice of specific pathogen-free (SPF) class, weighing between 16 to 22 grams, were purchased from the experimental animal center of Xiangya School of Medicine (Changsha, Hunan). Mice were assigned on random into four groups, with 14 mice in each group: ovalbumin group (OVA group; mice were stimulated with OVA to induce allergic asthma), OVA + si-PTEN group (mice were stimulated with OVA to induce allergic asthma and treated with si-PTEN plasmids), normal saline group (NS group; mice were treated with normal saline) and NS + si-PTEN group (mice were treated with normal saline and si-PTEN plasmids). The asthmatic mouse model was established by treating the mice with OVA. Mice were initially sensitized on Days 0, 7 and 14 by an intraperitoneal injection of 200 µL sensitizing liquid containing 20 µg OVA along with 2 mg of aluminum hydroxide gel in the OVA and OVA + si-PTEN groups. Mice in the OVA + si-PTEN group were received an intraperitoneal injection of 200 µL si-PTEN plasmids (Invitrogen Inc., Carlsbad, CA, USA). 21 days after the first sensitization, mice were exposed to 1% OVA aerosol for 6 to 8 weeks, 5 times a week, 30 minutes each time. On Days 0, 7 and 14, the mice of the NS and NS + si-PTEN groups were intraperitoneally injected 200 µL of normal saline. Mice in the NS + si-PTEN group were intraperitoneally injected with 200 µL si-PTEN plasmids (Invitrogen Inc., Carlsbad, CA, USA). On day 21, mice in the NS and NS + si-PTEN groups were administered 200 µL normal saline for 6 to 8 weeks, 5 times a week, 30 minutes each time.
Masson staining and observation of structure under microscope
The mice were euthanized by cervical dislocation, 24 hours after the last administration. Lung tissues were extracted and immediately fixed in 10% neutral formalin overnight and embedded in paraffin. The lung tissues were sliced into sections, after which Masson staining was performed. After de-paraffinization, the sections were stained with hematoxylin for 3 minutes, followed by a reaction with 1% hydrochloric acid-ethanol for 5 seconds, after which the color changed back to blue in water for 1 minute. The sections were separately rinsed with water. After completion of these steps, the sections underwent Ponceau fuchsin staining for 3 minutes at room temperature, and then the sections were treated with phosphomolybdic acid for about 5 minutes without washing. Then the lung sections were directly dyed with aniline blue for 5 minutes, followed by 1% acetic acid treatment for 1 minute, after which they were dehydrated by 95% alcohol and anhydrous ethanol dehydration 3 times. Sections were dried and sealed with neutral gum. Under a light microscope, collagen fibers appeared to be blue, the nuclei were dark blue, and smooth muscle fibers and red cells appeared red. Five random high power fields (HPF) of view were selected for visualization with morphological integrity and clear edge, and a diameter of approximately 200 µm in the transverse section of the bronchial cross-section. Image-Pro Plus 6.0 image analysis software was employed in order to analyze the inner perimeter of the bronchial wall (Pi), inner area of the bronchial wall (WAi), airway smooth muscle area (WAm), and the number of smooth muscle nuclei (N). The ratio of the WAi/WAm and N/Pi was considered as the standard measurement value, representing the bronchial wall thickness (WAi/Pi) and the smooth muscle thickness (WAm/Pi) and smooth muscle nuclei (N/Pi).
Immunohistochemical detection
After the sections were deparaffinized, they were boiled in 3% hydrogen peroxide to inactivate endogenous peroxidase, along with the addition of goat serum sealing liquid and the polyclonal antibody in BALB/c mice (Sigma-Aldrich, St. Louis, MO, USA). The bronchus was observed under a light microscope (Olympus DP80). Three HPF was randomly selected from each section (×200) with a diameter of about 200 µm approximately and were photographed and preserved for further analysis. Image-Pro Plus 6.0 image analysis software was used to measure the area of alpha smooth muscle actin (α-SMA+) (Sα)/bronchial basement membrane perimeter (Pb) (µm2/µm).
Isolation and culture of ASMCs
Under sterile conditions, using surgical scissors and forceps the small bronchus of pulmonary lobe or segment was carefully isolated from the mice in the NS and OVA groups. After removal of the epithelium, connective tissue, blood vessels and cartilages, tissues were washed with cold phosphate buffer saline (PBS) 3 times, and stored in a sterilized small glass bottle. Two drops of bovine serum albumin (BSA) were added and the smooth muscle was finely chopped into minced muscle blocks with a pair of iris scissors, which were then inoculated at the bottom of the culture bottle. Tissue were then incubated at 37 °C and 5% CO2 in an incubator, the bottle was inverted. Two hours later, the tissue blocks adhered to the wall, and DMEM medium containing 10% BSA was added. The medium was replaced once every 3 to 4 days. Approximately 14 days after, the cells appeared to be in a “peak and valley” structure due to fusion before undergoing 0.125% trypsin digestion. The cultured cells were passaged at 1:2 ratio and the cells from fourth to eight generations were used for subsequent experiments.
Grouping and transfection
ASMCs in the NS group were divided between the NS group (not transfected with any plasmid) and NS + si-PTEN group (transfected with si-PTEN plasmid). ASMCs in the OVA group were grouped into the OVA group (not transfected with any plasmid) and OVA + si-PTEN group (transfected with si-PTEN plasmid). In accordance with the instructions of the Lipofectamine 2000 kit (Invitrogen Inc., Carlsbad, CA, USA), Lipofectamine 2000 transfection reagent was mixed with oligonucleotide and allowed to incubate for 15 minutes, after which it was added to a 24 well-plate for culture and the culture medium was replaced after 6 hours. After culturing for 24 hours, the transfection plasmid was repeated once.
Quantitative reverse transcription PCR analysis
Fluorescent quantitative RT-PCR was performed using an Applied Biosystems 7300 reverse transcription PCR System and SYBR Premix Ex TaqTM (Takara Holdings Inc., Kyoto, Japan). After transfection, the total RNA from the cells in each group was extracted and reverse transcribed at 37 °C for 15 minutes and 5 seconds at 85 °C. The PTEN gene sequence used in this experiment was acquired from the NCBI (National Center for Biotechnology Information, MD, US) Nucleotide database. The primers were designed by an online design program. Homology search was performed using the Blast program in the GenBank database to check the sequence, and no homology sequence was found. The forward primer sequence of PTEN was 5'-TTGGCGGTGTCATAATGTCT-3', and the reverse primer sequence was 5'-GCAGAAAGACTTGAAGGCGTA-3' with a PCR product length of 450 bp. The primer sequences for the GAPDH-R internal reference were 5'-GTGGCAAAGTGGAGATTGTTGCC-3' (forward) and 5'-GATGATGACCCGTTTGGCTCC-3 (revere).
Western blotting
A BCA kit (Boster Bioengineering Co., Ltd., Wuhan, China) was used to measure the protein produced in tissues by RT-PCR. After measuring the protein concentration, 30 µg of the protein sample was subjected to electrophoresis in 10% SDS-polyacrylamide gel after boiling for 10 minutes at 95 °C and then the sample was transferred onto a PVDF membrane. The electrophoresis voltage was set between 80 to 120 V. The wet transfer was set at 100 mV for 45 to 70 minutes. After blocking for 1 hour under 5% BSA at room temperature, the membranes were incubated at 4 °C overnight with primary antibody (1:1,000; Cell Signaling Technologies, Beverly Mam, USA), and washed by TBST buffer thrice in which each wash took 5 minutes. Then the secondary antibody was added to the protein sample and allowed to incubate for an hour followed by a 5-minute wash, three times. Proteins were visualized with an enhanced chemiluminescence reagent (Amer-sham Pharmacia Biotech., Piscataway, NJ, USA) and exposed to film. β-actin could be used as a reliable internal reference. Bio-Rad Gel DocTM EZ imager (Bio-Rad Laboratories, Hercules, CA, USA) was used for the aforementioned. Image J software was employed in order to analyze the grey value.
CCK-8 assay
CCK-8 assay was performed according to the instructions provided by Dojindo Laboratories, Kumamoto, Japan. 100 µL mice ASMC suspension was isolated and transferred to a 96-well plate. The ASMC suspension was cultured separately for 0, 24, 48, 72 and 96 hours, after which 10 µL CCK-8 was added to each well. Cells were further cultured for 1 to 4 hours and then the wavelength was measured at 450 nm by a spectrophotometer. The CCK-8 kit was used to detect the percentage of proliferation when the mice ASMCs were transfected after 24, 48, 72, and 96 hours.
Flow cytometry
Two wells (on a 6-well plate) of cells in each group were used to prepare 1×106 cells/mL single cell suspensions which were then fixed with 4% paraformaldehyde at room temperature for 40 minutes. The cells underwent centrifugation at 1,000 r/min for 5 minutes followed by removal of fixation fluid. The cells were washed once with PBS after the supernatant was removed and were treated with 0.25% TritonX-100 on ice for 5 minutes. After washing the cells, 200 µL primary mouse A-SMA antibody (1:100) was added to the cells and incubated at 4 °C overnight. After washing the cell suspension twice, the secondary antibody goat anti-mouse IgG2a-FITC (1:50; 200 µL) was added to the cells and incubated at room temperature for 30 minutes. After centrifugal washing twice, 50 µL 0.1% Rnase was added and allowed to incubate for 10 minutes at room temperature. After incubation, 200 µL Propidium iodide (PI) (250 µg/mL) was added to the cell mixture and allowed to incubate devoid of light for 30 minutes. Flow cytometry was employed to examine and analyze the cell cycle. EXPO2 software (Beckman-Coulter, Krefeld, Germany) was used to obtain 10,000 FITC positive ASMCs in each sample, and the ASMCs cycle distribution was analyzed by MultiCycle DNA software (Phoenix, San Diego, CA, USA).
Statistical analysis
The acquired data was analyzed by SPSS 18.0 statistical software. Measurement data were presented as mean with standard deviations (SD). The t-tests were employed for comparing between two groups abiding normal distribution. The enumeration data were expressed as percentages and ratios, and were verified using the chi-square tests. P values less than 0.05 were considered to be statistically significant.
Results
OVA or silenced PTEN could thicken tracheal wall and the smooth muscle layer
In mice of the NS group, the bronchial structure appeared to be normal; the mucosal epithelium was intact with no obvious inflammatory reaction in the surrounding was observed under a light microscope. In the OVA group and OVA + si-PTEN group, the tracheal wall appeared to be thickened, with lumen stenosis and mucous plug. Furthermore, the airway mucosal folds increased and epithelial cells were necrotic and exfoliated. A lot of inflammatory cell infiltration was visible in the tracheal cell sections. In the submucosa, a lot of collagen deposits were clearly visible. In addition, there was a certain degree of thickening of airway wall and smooth muscle layer in the NS + si-PTEN group (Figure 1). The tracheal wall and the smooth muscle layer of the OVA, NS + si-PTEN and OVA + si-PTEN groups appeared to be significantly thicker and contained more smooth muscle cells than the NS group (P<0.05) (Table 1). The α-smooth muscle actin (α-SMA+) staining of the OVA group (5.29±0.54), NS + si-PTEN group (5.48±0.68) and OVA + si-PTEN group (8.65±0.75) was markedly enhanced compared to the NS group (2.35±0.39) (Figure 1).
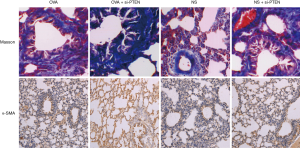

Full table
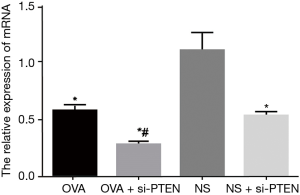
OVA could decrease PTEN mRNA expression compared with NS
The mRNA expression of PTEN in the OVA group significantly decreased compared with the NS group (P<0.05). The mRNA expression of PTEN in the OVA + si-PTEN group was the lowest amongst all groups, and significantly lowers than that of the OVA group (P<0.05). The mRNA expression of PTEN in the NS + si-PTEN group was significantly lower than the NS group. No such significant changes were observed in PTEN mRNA expression in between the OVA and OVA + si-PTEN groups (Figure 2).
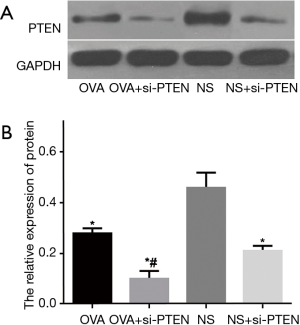
OVA could decrease PTEN protein expression compared with NS
The protein expression of PTEN of the OVA, OVA + si-PTEN and NS + si-PTEN groups was significantly lower than the NS group (P<0.05) (Figure 3). At the same time, the protein expression of PTEN was lower than that of the reference GAPDH in the OVA, OVA + si-PTEN and NS + si-PTEN groups, regardless of the fact that the protein expression of PTEN in the NS group was similar to that of GAPDH. The results of Image J software image analysis and quantitative analysis revealed that the OVA + si-PTEN group had a significantly decreased protein expression of PTEN compared with the OVA group (P<0.05).
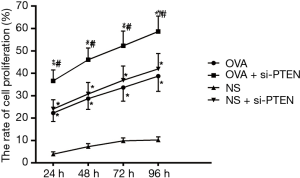
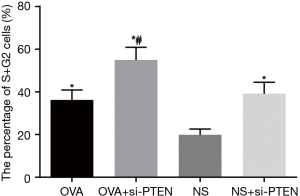
Silencing PTEN increases cell proliferation and more cells arrested in S and G2 stage while less cells arrested in the G1 stage
CCK-8 assay was used to analyze the ASMCs growth and proliferation. Data is shown in Table 2 and the cell growth curve is illustrated in Figure 4. Except for the NS group, the other three groups exhibited increased cell proliferation but no significant difference was observed among them in cell proliferation. Flow cytometry results showed that the proportion of cells in G1 stage decreased whereas the percentages of cells in S stage as well as G2 stage increased after PTEN gene silencing. Cells in S and G2 stages completed DNA replication and entered the mitotic stage. These results helped confirmed that the rate of cell division was faster and cell growth was accelerated. As shown in Figure 5, the cells in S and G2 stages of the NS group accounted for 19.87%±3.39%, those in the OVA group accounted for 36.21%±4.72%, cells in the OVA + si-PTEN group accounted for 54.98%±6.58%, and NS + si-PTEN group accounted for 39.16%±6.01%, respectively. In comparison with the NS group, there was a significant difference in the cells in S and G2 stages among the OVA, OVA + si-PTEN and NS + si-PTEN groups (P<0.05). The OVA + si-PTEN group had more cells in S and G2 stages compared with the OVA group (P<0.05). The results suggested that silencing PTEN might regulate cell cycle to accelerate proliferation of ASMCs.

Full table
Discussion
Allergic asthma is one of the most common diseases experienced by children in western countries (20). Presently, PTEN has been well established and has been associated to play an important role in the occurrence and development processes of different tumors (21). In this study, we conducted a series of experiments in order to investigate the relationship between PTEN gene silencing and ASMCs proliferation as well as airway remodeling in an allergic asthmatic mouse model. The results obtained from our study demonstrated that PTEN gene silencing could promote ASMC proliferation and airway remodeling.
PTEN silencing has shown to significantly thicken the tracheal wall and smooth muscle layer along with increasing α-SMA+ in mice with allergic asthma. It has been reported that thickening of the bronchial smooth muscle wall and increase in the number of infiltrating cells are some of the pathological changes experienced by asthmatic people, which is in consistency with our findings (22). Airway inflammation and airway remodeling are the main pathological features of allergic asthma (4). Airway remodeling is a result of a complex series of structural changes, primarily marked by smooth muscle hypertrophy and cell proliferation (23). PTEN gene is expressed endogenously in ASMCs, acting as a nodal regulator for multiple signaling pathways affecting cell size, cell proliferation and survival and then ultimately leading to inhibited proliferation and migration of cells (24). In addition, TGF-b-induced activation of fibroblasts into α-SMA-positive myofibroblasts in order to synthesize type-1 collagen is a vital event in allergic asthma (22). In line with our results, a prior study also observed that PTEN overexpression resulted in a reduced expression of α-SMA (25).
The results of our study showed that the PTEN mRNA and protein expressions were substantially lower in the OVA group, the OVA + si-PTEN group, and the NS + si-PTEN group compared with the NS group. The Loss-of-function model is a vital and informative method to characterize the pathological and physiological functions of mammalian genes (26). PTEN gene silencing is employed to inactivate the gene and promote the development of allergic asthma by inhibiting gene expressions (27). A previous study revealed that PTEN gene silencing elevates p-Akt levels and aberrantly interacted with p53, which consequently increases the risk of various cancers or developmental defects (28). A prior research showed a reduced expression of PTEN protein in 67 cases out of 236 breast carcinomas. Patients who exhibited lowered PTEN protein expression had a worse prognosis compared to the patients with a regular PTEN protein expression (29). More importantly, it has been reported that changes in PTEN levels are associated to the pathogenesis of allergic asthma (30). PTEN has been found to inhibit airway inflammation and airway hyperresponsiveness, both of which are fundamental features of allergic asthma (31,32). In consistency with our results, PTEN expression has been shown to decrease in murine asthma models (6).
On analyzing the rates of ASMC proliferation, it was observed that each group exhibited different degrees of ASMCs proliferation after PTEN silencing except for the NS group. The cell cycle changed whereby the proportion of cells in G2 and S stages was notably higher. Recent data demonstrated that PTEN affects cell invasion and gene expression by targeting its lipid phosphatase activity (33). Furthermore, PTEN has been suggested to play an important role in arresting the cell cycle in G1 stage and apoptosis, as well as regulating cell adhesion, differentiation and migration (34). To be specific, one previous study observed that more cells arrested in G0/G1 phase and suppressed cell proliferation were correlated with PTEN overexpression (35). A study conducted by Ressel et al. also clarified that PTEN gene could stimulate cell survival and proliferation via the B pathway of protein kinase through antagonizing the activity of phosphatidylinositol 3 kinase and loss of PTEN expression was related with poor prognosis (36). Great understanding of different mechanisms underlying PTEN gene regulation is beneficial for the diagnosis and treatment of patients (37).
To conclude, our study provides evidence suggesting that PTEN gene silencing can promote ASMC proliferation and airway tissue remodeling. Under these experimental conditions, gene duplication events or paralogs may be affected due to limitation of various factors leading to some inaccurate results. Our next step towards validating the credibility of PTEN gene silencing would be to move on from animal models and conduct further correlative study in clinical trials. It will be exciting to examine the interaction between PTEN gene and allergic asthma in further studies involving real patients and we hope to achieve significant results.
Acknowledgements
We thank the reviewers for critical comments.
Funding: This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); the 2016 “333 Project” Award of Jiangsu Province, the 2013 “Qinglan Project” of the Young and Middle-aged Academic Leader of Jiangsu College and University, the National Natural Science Foundation of China (81571055, 81400902, 81271225, 31201039, 81171012, and 30950031), the Major Fundamental Research Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (13KJA180001), and grants from the Cultivate National Science Fund for Distinguished Young Scholars of Jiangsu Normal University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was performed with approval of the animal ethics association of the Affiliated Municipal Hospital of Xuzhou (permit number: 201603002), and all efforts were made to minimize animal suffering.
References
- Staab EB, Sanderson SD, Wells SM, et al. Treatment with the C5a receptor/CD88 antagonist PMX205 reduces inflammation in a murine model of allergic asthma. Int Immunopharmacol 2014;21:293-300. [Crossref] [PubMed]
- Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem 2011;286:32883-9. [Crossref] [PubMed]
- Gibeon D, Menzies-Gow A. Recent changes in the drug treatment of allergic asthma. Clin Med (Lond) 2013;13:477-81. [Crossref] [PubMed]
- Zhang WX, Li CC. Airway remodeling: a potential therapeutic target in asthma. World J Pediatr 2011;7:124-8. [Crossref] [PubMed]
- Bosnjak B, Stelzmueller B, Erb KJ, et al. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res 2011;12:114. [Crossref] [PubMed]
- Lan H, Zhong H, Gao Y, et al. The PTEN tumor suppressor inhibits human airway smooth muscle cell migration. Int J Mol Med 2010;26:893-9. [PubMed]
- Naylor B. The shedding of the mucosa of the bronchial tree in asthma. Thorax 1962;17:69-72. [Crossref] [PubMed]
- Roche WR, Beasley R, Williams JH, et al. Subepithelial fibrosis in the bronchi of asthmatics. Lancet 1989;1:520-4. [Crossref] [PubMed]
- Elias JA, Zhu Z, Chupp G, et al. Airway remodeling in asthma. J Clin Invest 1999;104:1001-6. [Crossref] [PubMed]
- Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med 1997;156:229-33. [Crossref] [PubMed]
- Carroll N, Elliot J, Morton A, et al. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993;147:405-10. [Crossref] [PubMed]
- Jácome AA, Coutinho AK, Lima EM, et al. Personalized medicine in gastric cancer: Where are we and where are we going? World J Gastroenterol 2016;22:1160-71. [Crossref] [PubMed]
- Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol 2001;14:672-6. [Crossref] [PubMed]
- Huang G, Redelman-Sidi G, Rosen N, et al. Inhibition of mycobacterial infection by the tumor suppressor PTEN. J Biol Chem 2012;287:23196-202. [Crossref] [PubMed]
- Chen G, Tang J, Ni Z, et al. Antiasthmatic effects of resveratrol in ovalbumin-induced asthma model mice involved in the upregulation of PTEN. Biol Pharm Bull 2015;38:507-13. [Crossref] [PubMed]
- Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci 2001;114:2375-82. [PubMed]
- Govatati S, Kodati VL, Deenadayal M, et al. Mutations in the PTEN tumor gene and risk of endometriosis: a case-control study. Hum Reprod 2014;29:324-36. [Crossref] [PubMed]
- Byun DS, Ahmed N, Nasser S, et al. Intestinal epithelial-specific PTEN inactivation results in tumor formation. Am J Physiol Gastrointest Liver Physiol 2011;301:G856-64. [Crossref] [PubMed]
- Aravamudan B, Thompson M, Pabelick C, et al. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med 2012;16:812-23. [Crossref] [PubMed]
- Lawson JA, Rennie DC, Cockcroft DW, et al. Childhood asthma, asthma severity indicators, and related conditions along an urban-rural gradient: a cross-sectional study. BMC Pulm Med 2017;17:4. [Crossref] [PubMed]
- Li HG, Liu FF, Zhu HQ, et al. Association of PTEN gene polymorphisms with liver cancer risk. Int J Clin Exp Pathol 2015;8:15198-203. [PubMed]
- Stumm CL, Halcsik E, Landgraf RG, et al. Lung remodeling in a mouse model of asthma involves a balance between TGF-beta1 and BMP-7. PLoS One 2014;9:e95959. [Crossref] [PubMed]
- Yang YG, Tian WM, Zhang H, et al. Nerve growth factor exacerbates allergic lung inflammation and airway remodeling in a rat model of chronic asthma. Exp Ther Med 2013;6:1251-58. [Crossref] [PubMed]
- Guha M, Plescia J, Leav I, et al. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res 2009;69:4954-8. [Crossref] [PubMed]
- Xie B, Zheng G, Li H, et al. Effects of the tumor suppressor PTEN on the pathogenesis of idiopathic pulmonary fibrosis in Chinese patients. Mol Med Rep 2016;13:2715-23. [Crossref] [PubMed]
- Gutschner T, Baas M, Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res 2011;21:1944-54. [Crossref] [PubMed]
- Zhang C, Yang C, Whitham SA, et al. Development and use of an efficient DNA-based viral gene silencing vector for soybean. Mol Plant Microbe Interact 2009;22:123-31. [Crossref] [PubMed]
- Zou S, El-Hage N, Podhaizer EM, et al. PTEN gene silencing prevents HIV-1 gp120(IIIB)-induced degeneration of striatal neurons. J Neurovirol 2011;17:41-9. [Crossref] [PubMed]
- Tsutsui S, Inoue H, Yasuda K, et al. Inactivation of PTEN is associated with a low p27Kip1 protein expression in breast carcinoma. Cancer 2005;104:2048-53. [Crossref] [PubMed]
- Sharma M, Batra J, Mabalirajan U, et al. A genetic variation in inositol polyphosphate 4 phosphatase a enhances susceptibility to asthma. Am J Respir Crit Care Med 2008;177:712-9. [Crossref] [PubMed]
- Baelder R, Fuchs B, Bautsch W, et al. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J Immunol 2005;174:783-9. [Crossref] [PubMed]
- Kwak YG, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest 2003;111:1083-92. [Crossref] [PubMed]
- Tibarewal P, Zilidis G, Spinelli L, et al. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal 2012;5:ra18. [Crossref] [PubMed]
- Han L, Zhang AL, Xu P, et al. Combination gene therapy with PTEN and EGFR siRNA suppresses U251 malignant glioma cell growth in vitro and in vivo. Med Oncol 2010;27:843-52. [Crossref] [PubMed]
- Luo L, Gong YQ, Qi X, et al. Effect of tumor suppressor PTEN gene on apoptosis and cell cycle of human airway smooth muscle cells. Mol Cell Biochem 2013;375:1-9. [PubMed]
- Ressel L, Millanta F, Caleri E, et al. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol 2009;46:860-8. [Crossref] [PubMed]
- Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci 2007;120:4071-9. [Crossref] [PubMed]



