Independent long-term result of robotic thymectomy for myasthenia gravis, a single center experience
Introduction
Myasthenia gravis (MG) is a rare autoantibody-mediated, neuromuscular junction suffered, autoimmune disease, with a prevalence rate of 77.7 cases per million worldwide (1). The history of thymectomy as an important therapy for MG started 80 years ago when Blalock et al. reported the relationship between thymus excision and remission of MG (2). In the following decades, surgical approach for MG developed prosperously all over the world, such as transsternal thymectomy, transcervical thymectomy, subxiphoid thymectomy, and thoracoscopic (either thoracoscopic or robotic) thymectomy. However, prospective randomized trial has long been lacked worldwide, until Wolfe et al. published their results of a multicenter randomized trail (3) in last year, confirming improvement clinical outcomes by thymectomy for patients with nonthymomatous MG. It was a great stride for surgical treatment of MG, but there still remains several questions with regards to the candidates, the optimal surgical approach, the timing and the extent of resection (4,5).
Representing the newest minimal invasive technique, robotic thymectomy has been increasing rapidly worldwide in recent years, as it provides with multiple advantages with less trauma comparing to the open procedure (6-8). The long-term results of robotic thymectomy for MG have also been published by several investigators (9-11). Whereas, immunotherapy as the mainstay of treatment for MG, it is frequently given to thymectomized patients to maintain pharmacologic remission (PR) nowadays (12-15). The usage of immunosuppressants would certainly play a role in the overall remission rate of thymectomized MG patients (16). However, few investigations of robotic thymectomy for MG has separated the confounding influence of immunotherapy when analyze the long-term results. Thus, the actual independent effect of robotic thymectomy for MG patients is unclear.
The present report introduces our observation of the relatively independent long-term results after robotic thymectomy for MG.
Methods
A retrospectively study was performed for patients who diagnosed as MG and underwent robotic extended thymectomy with da Vinci robotic system (Intuitive Surgical, Inc, Sunnyvale, CA, USA) in department of thoracic surgery of PLA Rocket Force General Hospital between May 2009 and December 2012. The study was approved by the medical ethics committee of PLA Rocket Force General Hospital (approval number KY2017027), and informed consent was got from each patient. The diagnosis of MG was based on histories, clinical features, and laboratory examination including repetitive nerve stimulation test, edrophonium chloride test, acetylcholinesterase antibody (AbAchR) and muscle-specific tyrosine kinase (MUSK) level. The classification of MG and the postintervention status were evaluated mainly according to the classification of the Myasthenia Gravis Foundation of America (MGFA) (17). The clinical absolute and relative scoring system (CARSS) (18,19) were adopted to assess the effect of therapy. Preoperative evaluation included a chest computer tomography (CT) scan, an electrocardiogram (ECG), cardiopulmonary function, and serological antibody test. Patients with ocular MG (OMG) were enrolled in case of thymic hyperplasia or thymoma suggested by radiology. Patients with insufficient cardiopulmonary capacity to undergo single-lung ventilation, serum antibody-MUSK positive and radiological evidence of invasive thymoma served as the exclusion criteria (11,20).
Surgical technique
General anesthesia and a double-lumen tube intubation with single lung ventilation were carried out. Routinely, the right-side approach was preferred aiming of minimizing the interference of the heartbeat. The left-side approach was selected if the majority of thymus gland or the thymoma located on the left side of mediastinum. The patient was placed supine with the thoracic cavity of surgical approach lifting up 30, and the homolateral shoulder holding down as much as possible to facilitate the movement of the robotic arm (Figure 1A).
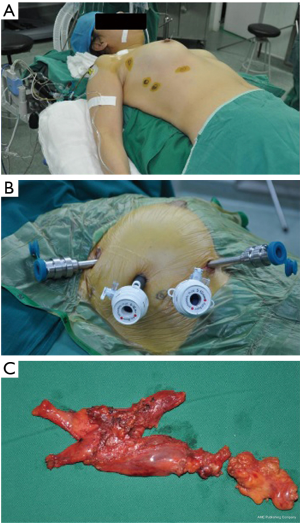
Generally, 4 trocars were placed, among which 3 trocars were connected with the 3 robotic arms of the table cart, and the fourth one manipulated by the assistant (Figure 1B). The first 5 mm trocar located in the sixth intercostal space between the anterior axillary and the mid-axillary line, and was introduced with the imaging system. Then, CO2 insufflation started with pressure ranging from 6 to 10 mmHg (21). Under direct vision by endoscopic imaging system, the second and third trocars were introduced in the third and fifth (or sixth) intercostal space, about a handbreadth left or right of the camera trocar, as described in our previous Chinese publication (22). The last trocar was introduced to place the attractor and the aspirator.
The dissociation procedure mainly operated with a bipolar electrocoagulation and a coagulation hook. The thymic and perithymic tissue were en-bloc resected, ranging from the innominate vein superiorly to the diaphragm inferiorly and between bilateral phrenic nerves as described in the existing literature (7,9,11). Briefly, opened the mediastinal pleura below the sternum, dissociated the thymus and peri-thymic tissue, cut off the thymic veins with electrocoagulation or ultrasonic scalpel, separated the thymic bipolar and thyreoidea inferior, finally, removed the specimen (Figure 1C) in an endobag through the lengthened incision in the fifth intercostal space. In case of indication of a thymoma preoperatively, the whole procedure of excision would under the principle of “no-touch” dissection technique (23). The innominate vein and the bilateral phrenic nerves were seriously protected. After extraction of the specimen, inspection of the operation field, and inflation of the atelectasis, a 26 F chest tube was inserted through the incision of the camera trocar, and the surgical procedure completed.
Medical treatment
The medical therapy was mainly implemented by a skilled neurologist. The usage of cholinesterase inhibitors (CH) was modulated to less than 180 mg/day for patients in need before operation, and began with a minimal dose during the immediate postoperative, then gradually increased for prevention of cholinergic crisis (24). Intravenous immunoglobulin (IVIG) and plasma exchange therapy would be temporarily adopted for MG crisis. The short-term effect after surgery was quantitatively assessed by the clinical relative score (CRS) regularly, which initially proposed by Professor Xian-Hao Xu and defined as (pretreatment absolutely score-posttreatment absolutely score)/pretreatment absolutely score (18,19). The principle of immunosuppressive (IM) therapy was based on the MGFA classes and the CRS. Generally, for MGFA classes I, sequential observation was recommended unless status aggravated; for other MGFA classes, IM therapy was recommended if the CRS <25% during the postoperative period. Prednisone and other IM agents including tacrolimus, cyclosporine and cyclophosphamide were administrated based on clinical medication guideline. The IM agents usually started with a lower dose and increased gradually according to the concentration of blood drug then sustained. When the patient’s CRS reached 80–95% for at least 3 months, the CH reduced gradually. Then the IM agents tapered after the discontinuation of the CH.
Follow-up
The data of clinical feature, surgical procedure (e.g., conversion, blood loss, and operative time), perioperative complications, morbidity/mortality and the post-operative medical therapy were documented in detail. The finally postintervention status, on the basis of MGFA recommendations (17), were calculated as complete stable remission (CSR), PR, minimal manifestations (MM) and changes in status (improved, unchanged, worse, exacerbation, and died of MG), and significant improvement is defined as the total of CSR, PR, and MM.
Statistical analysis
All the data was recorded and analyzed with SPSS22.0 statistical software. Data were expressed as absolute numbers, percentage, median or mean values ± standard deviation (SD). Baseline characteristics of the patients with or without IM usage were compared using Fisher’s exact test for categorical variables and the Wilcoxon test for continuous variables. Kaplan-Meier method and log-rank test were used to univariate analysis of the probabilities cumulative CSR and significant improvement. The COX regression was performed for multivariate analysis. P value <0.05 was considered as statistical significant.
Results
During the observation period, data of 37 cases was available with a mean follow-up of 70.0 months. The median age was 40 years (range from 18 to 65 years), and the median preoperative duration of symptoms was 12 months (range from 1 to 96 months). All the robotic procedure completed successfully with no conversion to thoracotomy and no operative death. The mean operation time was 128.9 min, and the mean blood lose was 77.6 mL. Complications occurred in 3 cases (8.1%), including chylothorax (1, 2.7%), myasthenia crisis (1, 2.7%), and acute laryngitis (1, 2.7%), and all recovered by conservative treatment.
During the postoperative period, 12 (32.4%) patients kept no usage of IM therapy till the last follow, whereas 25 (67.6%) patients accepted IM therapy, as shown in Table 1. The differences of the baseline characteristics were not significant except for a trend of gender. Most of IM therapy (23 cases) started within a week or so after operation, whereas minority patients (2 cases) underwent salvage IM therapy 2 years later due to aggravation. The detail of medical treatment was shown in Table 2.
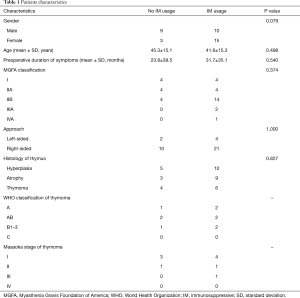
Full table
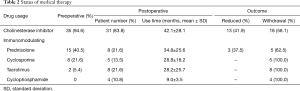
Full table
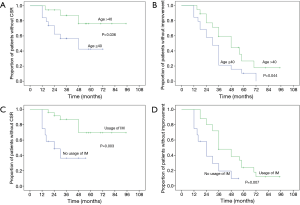
The overall 5-year probability of CSR rate was 40.6% and improvement rate was 81.6%. Probable impact factors of 5-year CSR and improvement by log-rank and COX regression analysis were shown in Table 3. Multivariate analysis showed that, the long-term remission rate differed significantly when grouping by patient age and usage of IM therapy postoperatively. Cumulative remission analysis by Kaplan-Meier method was shown in Figure 2. The young patients (age ≤40) displayed a significant better cumulative CSR (P=0.015) and a trend of better improvement rate (P=0.050) compared to the elder patients (age >40). Patients independent of IM postoperatively showed a significant better CSR rate (P=0.014) and improvement rate (P=0.024) compared to those in need of IM usage. The lower MGFA classes had a trend of higher remission rate, but the differences were not significant by multivariate analysis. No significant differences were found for the remission rate in terms of gender, pathology and the duration of symptoms.
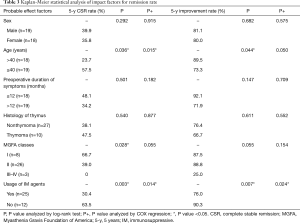
Full table
Discussion
The effectiveness of thymectomy for MG has been widely accepted worldwide. Robotic thymectomy, one of the most innovative approaches, has been verified associating with favorable short and long-term outcomes by several investigations (6,9,10,25). Several advantages of the robotic system, such as the 3D quality of visualization, the superior maneuverability of the surgical instruments, and the filter of hand tremors, made it best fit for operation in tiny anatomic regions, just like anterior mediastinum where the thymus located (26). And these technologic superiority may convert to a radical resection of the thymus and the adipose tissues, and finally result in a better disease remission for MG (27). Our study also confirmed the safety and facility of robotic thymectomy, in terms of no conversion, short operation time, low blood loss and complication rate (<10%). Usually we prefer to place four trocars during surgical procedure, as this trocar was beneficial for exposure of the operation field, elimination of the smog and emergency in case to our experiences.
Due to the fluctuating nature of the disease, long-term results might be not in line with the short-term results (28), therefore long-term effect of robotic thymectomy for MG have been paid more attention. According to literature, the 5-year CSR rate ranges from 16.9% to 42% (10,29). Whereas, approximately 20% of patients would undergo spontaneous clinical remission during an average of 5 years (30). In our series, the overall 5-year CSR and improvement rate after robotic thymectomy was 40.6% and 81.6%. The variation may be caused by several factors, such as the differences in patient characteristics (11), the surgical approach, the extent of thymus resection (31), and the usage of IM agents (16).
There remains controversial about the indication of thymectomy for OMG (32,33). However, the risk of generalization from OMG to generalized MG (GMG) do exist for part of patients, and certain treatment might delay or prevent the onset of GMG (1). Besides, the presence of OMG before operation was one of the favorable factors according to a large sample retrospective study (34). Therefore, 8 cases of MGFA classes I were enrolled in our study, among which 5 cases achieved CSR and all of the 8 patients achieved significant improvement till the last follow up. The MGFA classes I showed significant higher CSR rate than other classes by the log-rank test (P=0.028). However, by multivariate analysis, it only showed a trend of higher CSR rate (P=0.055), which may be limited by the relatively small samples.
Several researchers have reported, the younger implied a better clinical remission than the elder after operation, our results was almost in line with them (11,16,35). The young patients (age ≤40) had a significant higher CSR rate (P=0.015) and a trend of higher improvement rate (P=0.05) compared to the old (age >40) by COX multivariate analysis.
The IM therapy are frequently applied for thymectomized MG patients to maintain a PR (12,30,36). However, due to lack of randomized control trial, the usage of immunosuppressant varied and no uniform criterion of IM therapy was available for thymectomized MG patients (37). Thus, the IM therapy would probably impact on the results of surgical treatment for MG. To explore the independent long-term effect of robotic thymectomy, a period of sequential observation rather than immediate IM therapy was carried out after surgical procedure in our department. The assessment of surgical procedure was based on the CRS which proposed by Professor Xian-Hao Xu in 1990s and widely accepted all over China (18). The IM therapy was recommended for MGFA classes II–V (GMG) with CRS <25% postoperatively, otherwise, sequential observation was recommended till disease aggravated. After a mean follow up of 70.0 months, we found that, a significant higher 5-year CSR rate (63.5%) and improvement rate (90.3%) were showed for patients without IM usage compared to patients with IM usage. And the remission rate of patients with IM therapy in our series was the same as the previous literature (10,29). These indicated that, the monotherapy of robotic thymectomy could provide long-term CSR for a considerable proportion of patients, and in view of our principle of IM therapy, the timing of IM therapy might not impact much on the long-term results. Thus, a temperate delayed IM therapy for robotic thymectomized MG patients was feasible with satisfactory results, and could contribute to minimize the inevitable side-effects of immunosuppressant. In recent years, this principle of IM therapy has been also applied for patients underwent thymectomy by video-assisted thoracoscopic surgery (VATS), due to similar effectiveness and less cost might attain by VATS thymectomy according to the literature (26) and our previous experiences. The long-term effectiveness is under observation. The precise selection of patients who could benefit from the monotherapy of surgical procedure is of the most importance, and the individualized IM therapy should be a major subject for future research of MG. The available targets such as the handful of antibodies are far not sufficient, besides, the acetylcholine receptor (ACHR) antibody, as the most widely applied marker, dose not correlate well with the clinical severity (38). Thus, more promising investigations such as the researches on genetics and epigenetics targets for MG maybe of great prospective (39,40).
Limitations
Several limitations exist in the present study. First, as the low incidence of disease, the sample size was relatively small and not all MGFA classes were included, which could lead to a selection bias. Second, the history of medicine treatment before operation inevitably differed among patients, and this could lead to a confounding bias. Third, like most previous researches, the retrospective nature of the study couldn’t draw solid conclusions, thus big sample size observation and multi-center randomized controlled trials are urgently needed.
Conclusions
In conclusion, the present study indicated that, monotherapy of robotic thymectomy may lead to a favorable long-term result for part of MG patients. Individualized IM therapy should be taken into account for robotic thymectomized patients and further randomized controlled trails are expected.
Acknowledgements
The authors thank Dr. Ke-Cheng Zhang and Dr. Yong Zhao for help analyzing the data and image processing.
Funding: This work was supported by Natural Science Foundation of China (NSFC) (No. 81573026).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the medical ethics committee of PLA Rocket Force General Hospital (approval number KY2017027), and informed consent was got from each patient.
References
- Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol 2016;263:826-34. [Crossref] [PubMed]
- Blalock A, Harvey AM, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland. JAMA 1941;117:1529-33. [Crossref]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Marulli G, Rea F. Myasthenia gravis and thymectomy: many doubts and few certainties. Eur J Cardiothorac Surg 2015;48:46-7. [Crossref] [PubMed]
- Tzankov A. Commentary on “Randomized trial of thymectomy in myasthenia gravis”. J Thorac Dis 2016;8:E1420-E1422. [Crossref] [PubMed]
- Marulli G, Maessen J, Melfi F, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg 2016;5:18-25. [PubMed]
- Xu S, Liu X, Li B, et al. Robotic thoracic surgery of total thymectomy. Ann Transl Med 2015;3:156. [PubMed]
- Rea F, Schiavon M, Marulli G. Robotic thymectomy for myasthenia gravis. Ann Cardiothorac Surg 2015;4:558-60. [PubMed]
- Freeman RK, Ascioti AJ, Van Woerkom JM, et al. Long-Term Follow-Up After Robotic Thymectomy for Nonthymomatous Myasthenia Gravis. Ann Thorac Surg 2011;92:1018-22; discussion 1022-3. [Crossref] [PubMed]
- Keijzers M, de Baets M, Hochstenbag M, et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur J Cardiothorac Surg 2015;48:40-5. [Crossref] [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [Crossref]
- Ponseti JM, Gamez J, Azem J, et al. Post-thymectomy combined treatment of prednisone and tacrolimus versus prednisone alone for consolidation of complete stable remission in patients with myasthenia gravis: a non-randomized, non-controlled study. Curr Med Res Opin 2007;23:1269-78. [Crossref] [PubMed]
- Ponseti JM, Gamez J, Azem J, et al. Tacrolimus for Myasthenia Gravis: A Clinical Study of 212 Patients. Ann N Y Acad Sci 2008;1132:254-63. [Crossref] [PubMed]
- Sathasivam S. Current and emerging treatments for the management of myasthenia gravis. Ther Clin Risk Manag 2011;7:313-23. [Crossref] [PubMed]
- Spring PJ, Spies JM. Myasthenia gravis: options and timing of immunomodulatory treatment. BioDrugs 2001;15:173-83. [Crossref] [PubMed]
- Lindberg C, Andersen O, Larsson S, et al. Remission rate after thymectomy in myasthenia gravis when the bias of immunosuppressive therapy is eliminated. Acta Neurol Scand 1992;86:323-8. [Crossref] [PubMed]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [Crossref] [PubMed]
- Liu GC, Gao BL, Yang HQ, et al. The clinical absolute and relative scoring system-A quantitative scale measuring myasthenia gravis severity and outcome used in the traditional Chinese medicine. Complement Ther Med 2014;22:877-86. [Crossref] [PubMed]
- Wang XY, Xu XH, Sun H. A clinical absolute and relative score system for myasthenia gravis. Chin J Neurol 1997;30:87-90.
- Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis 2014;6:726-33. [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]
- Chen X, Han B, Yin DT, et al. Technical Points on Robotic and Video Assisted Thoracoscopic Thymectomy. Chinese Journal of Modern Operative Surgery 2012.
- Detterbeck FC. The international thymic malignancy interest group. J Natl Compr Canc Netw 2013;11:589-93. [Crossref] [PubMed]
- Jenkins LC, Chang J, Saxton GD. Myasthenia gravis: anesthetic and surgical management of the patient undergoing thymectomy. Can Med Assoc J 1965;93:198-203. [PubMed]
- Kawaguchi K, Fukui T, Nakamura S, et al. A bilateral approach to extended thymectomy using the da Vinci Surgical System for patients with myasthenia gravis. Surg Today 2018;48:195-9. [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Jaretzki A 3rd. Thymectomy for myasthenia gravis: analysis of controversies--patient management. Neurologist 2003;9:77-92. [Crossref] [PubMed]
- Roth T, Ackermann R, Stein R, et al. Thirteen years follow-up after radical transsternal thymectomy for myasthenia gravis. Do short-term results predict long-term outcome? Eur J Cardiothorac Surg 2002;21:664-70. [Crossref] [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic Thymectomy with the da Vinci Robotic System for Myasthenia Gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Gotterer L, Li Y. Maintenance immunosuppression in myasthenia gravis. J Neurol Sci 2016;369:294-302. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Suzuki Y. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg 1996;112:371-5. [Crossref] [PubMed]
- Murai H. Indication of thymectomy and immunotherapies on myasthenia gravis. Rinsho Shinkeigaku 2007;47:875-6. [PubMed]
- Yu S, Li F, Chen B, et al. Eight-year follow-up of patients with myasthenia gravis after thymectomy. Acta Neurol Scand 2015;131:94-101. [Crossref] [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853-9. [Crossref] [PubMed]
- Cruz JL, Wolff ML, Vanderman AJ, et al. The emerging role of tacrolimus in myasthenia gravis. Ther Adv Neurol Disord 2015;8:92-103. [Crossref] [PubMed]
- Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol 2008;4:317-27. [Crossref] [PubMed]
- Sanders DB, Burns TM, Cutter GR, et al. Does change in acetylcholine receptor antibody level correlate with clinical change in myasthenia gravis? Muscle Nerve 2014;49:483-6. [Crossref] [PubMed]
- Mamrut S, Avidan N, Truffault F, et al. Methylome and transcriptome profiling in Myasthenia Gravis monozygotic twins. J Autoimmun 2017;82:62-73. [Crossref] [PubMed]
- Molin CJ, Westerberg E, Punga AR. Profile of upregulated inflammatory proteins in sera of Myasthenia Gravis patients. Sci Rep 2017;7:39716. [Crossref] [PubMed]


