Metastatic hepatic clear cell carcinoma presenting as lump in the hilum of the lung: a case report and review of the literature
Introduction
Clear cell tumors are famous for their transparent cytoplasm under light microscope. They usually originate from the kidney and ovary, few can also be found in the thyroid, breast, endometrium, etc. The liver and lung clear cell carcinoma is rarely seen clinically. Lung metastatic hepatic clear cell carcinoma is extremely rare. Here we report a case of metastatic hepatic clear cell carcinoma presenting as lump in the hilum of the lung.
Case presentation
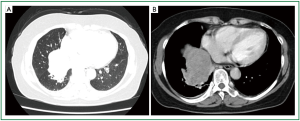
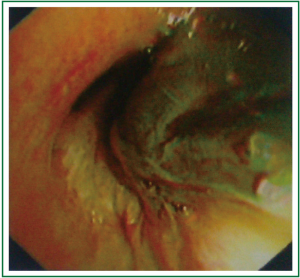
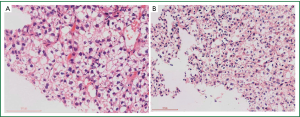
A 52-year-old Chinese woman was admitted with right chest pain, cough, expectoration and bloody phlegm of 3-month duration. She denied fever, dyspnea, night sweats, hemoptysis or weight loss. She was a chronic hepatitis B carrier and underwent left external lobectomy because of primary hepatic cell carcinoma in 2009. After operation, she received transcatheter arterial embolization treatment once. On physical examination, vital signs were normal. The right inferior lung showed flatness on percussion and no breath sound could be heard on auscultation. Crackles, wheeze and rhonchi were absent. She showed no clubbing fingers, nor did she have peripheral edema, rash, skin lesions or enlarged lymph nodes. Laboratory tests on admission showed no abnormality (liver function test and serum AFP level were normal on admission). Chest computer tomography (CT) revealed a 6.2 cm × 6.8 cm shallow lobulated irregular shaped mass in the hilum of the right lung, with thickening of bronchial wall and bronchostenosis of corresponding intermediate segmental bronchi (Figure 1). No mass lesion was identified in other organs such as bone, kidneys, adrenal glands, brain or gynecologic system. The whole body positron emission tomography/computed tomography (PET/CT) revealed a 7.5 cm × 9.4 cm mass involving the right inferior lung near the hilum, surrounded by abnormal radioactivity, conforming to the features of right inferior lung carcinoma with central necrosis and metastasis of right hilar lymph node. No obvious abnormalities were found in other parts of the body. Electronic bronchoscopy on August 21, 2012 showed that right intermediate bronchus was completely obstructed by a neoplasm, the mucous of right middle lobar bronchus was smooth, while right inferior lobar bronchus was poorly viewed. We failed to get any histopathology slide and performed electronic bronchoscopy again on August 28, 2012. The bronchoscopy revealed that the right inferior lobar bronchus was completely obstructed by obsolete blood clots (Figure 2). After clearing blood clots and hemostasis with adrenaline, it revealed that the mucous of right inferior lobar bronchus was obviously congested, swollen and rough. The medial basal segment was blocked, the distal end of lateral and posterior basal segments were still blocked, while the dorsal and anterior basal segment were unobstructed. We took the right lower basal segment for pathological examination. The light microscopy revealed some clear cells (Figure 3A), and immunohistochemistry showed negative results for CD10, CK7, RCC, TTF and Vimentin but positive results for CK-Pan. The pathological diagnosis was metastatic hepatic clear cell carcinoma. Besides, the pathological slides obtained by CT-guided lung biopsy also showed poorly differentiated carcinoma, the immunohistochemistry of which showed negative results for CD10, CK5/6, HMB45, P63, S-100, TTF and AFP but positive results for CK-Pan, hepatocyte and RCC. Considering the patient’s history, physical examination, bronchoscopy, and histopathology with immunohistochemical staining results, the final diagnosis was: lung metastatic hepatic clear cell carcinoma. The patient was treated with two cycles of paclitaxel and lobaplatin with three-week intervals. The chemotherapy was ceased, as she could not tolerate the side effects of chemotherapy. Unfortunately, the patient was subsequently lost to follow-up.
Discussion
Primary clear cell carcinoma of the liver (PCCCL) is a histological variant of primary hepatocellular carcinoma (HCC) that has been reported with low frequencies in the worldwide literature, which is pathologically characterized by diffuse clear cells of the tumor, showing a clear cytoplasm that does not stain with hematoxylin and eosin. The vacuolated appearance of the tumor cells is due to the cytoplasmic accumulation of large amounts of glycogen or lipid that are dissolved by routine histological processing (1). The mechanism for development of clear cells is presumed to involve metabolic disorders and abnormalities of sugar metabolism for reasons including decreased portal blood flow and underdeveloped tumor arteries in the early stage of cancer (2,3). The imaging characteristics of PCCCL are similar to those of HCC, including early enhancement and rapid washout of contrast agent on dynamic contrast scans, and presence of portal vein thrombus or tumor rupture. These imaging features may help differentiate PCCCL from other liver tumors, such as hemangioma and hepatic metastases (4). PCCCL is characterized by high male prevalence, high rate of association with hepatitis, and has no significant association with age, liver cirrhosis or serum AFP level (5). PCCCL can be confused with metastatic hepatic lesions of renal, ovarian, or adrenal origin. Immunohistochemical staining should be performed for both confirmation of diagnosis and establishment of differential diagnosis, which revealed EMA, HMB45, CK7, CK20, melan A and steroidogenic factor-1 negativity, and AFP, CK8, CK18, hepatocyte and hepatocyte paraffin 1 (Hep Par1) positivity (6). AFP can be negative or positive. Hepatocyte is hepatic-specific antigen, while Hep Par1 is a monoclonal antibody that reacts with hepatocytes, both of which can be used for differential diagnosis of hepatic tumors. Surgical resection is an effective treatment for patients with PCCCL and contributes to favorable outcomes and even long-time survival (7). Many studies reported that PCCCL had a better prognosis than other HCCs (8,9). Liu suggested that 1-, 3- and 5-year survival rates in PCCCL were significantly higher than in HCC (10). Ji suggested that Patients with high clear cell ratio had improved prognosis. Poor preoperative liver function, HCV infection, large vascular invasion, and multiple tumor occurrence are risk factors for metastasis and postoperative recurrence of PCCCL (11).
We reviewed literature about lung metastatic PCCCL, only Huang reported one case (12). The patient was diagnosed with PCCCL via fine needle aspiration biopsy of the liver. Multiple metastatic nodules were discovered three years after radiofrequency ablation and pulmonary metastatic PCCCL was confirmed via CT guided lung biopsy. Our case showed that the patient was initially diagnosed with primary hepatocyte carcinoma (Figure 3B) and underwent left external lobectomy. Two years afterwards, a shallow lobulated irregular shaped mass in the hilum of the right lung was revealed by CT, the immunohistochemistry of CT-guided lung biopsy showed positive results for hepatocyte, which demonstrates that the mass was originated from liver. Finally, the pathological diagnosis was lung metastatic hepatic clear cell carcinoma. The image characteristics of the mass in the lung resemble central lung cancer. It’s easy to misdiagnose without detailed history and immunohistochemical staining.
In this case, it’s necessary to differentiate metastatic hepatic clear cell carcinoma from primary pulmonary clear cell carcinoma. Pulmonary clear cell carcinoma is defined by the World Health Organization as anaplastic large cell tumor forming nests, clusters, and sheets of cells with large vesicular nuclei and an abundant clear or foamy cytoplasm, which may or may not contain glycogen (13). No mucin is present, and this category does not include adenocarcinoma or squamous carcinoma with clear cells. CT usually reveals a single nodule or mass at the outer side of the lung. Immunostaining is diagnostically useful, distinguishing pulmonary clear cell carcinoma from lung metastasis of renal clear cell carcinoma or clear cell squamous cell carcinoma. It reveals that the tumor cells express not only epithelial markers, including cytokeratin (CK) and epithelial membrane antigen (EMA), but also Ley, one of the stage-specific embryonic carbohydrate antigens. Vimentin is absent. However, the immunostaining of PCCCL shows positive expression of AFP, CK8, CK18, hepatocyte and Hep Par1. Periodic acid-schiff (PAS) staining of pulmonary clear cell carcinoma is negative or weakly positive, indicating that the carcinoma contains no or little glycogen. Both fat staining and Alician-blue staining are negative, indicating that the carcinoma contains no lipid or mucin. On the contrary, the cytoplasm of PCCCL is accumulated with large amounts of glycogen or lipid. In summary, for the differentiation of the two diseases, pathology and immunohistochemical staining are important in terms of confirming the diagnosis.
The present case exemplifies that not all metastatic lesions of the lung appear to be multiple, random distributed, round nodules at the outer side of the lung. As for patients with a history of malignant tumor, if the lesion in the lung is highly suspected of malignancy, it’s imperative to consider metastatic lesions besides primary lung carcinoma even when the lesion is at the hilum of the lung. This case also highlights the importance of repeated bronchoscopy as well as a thorough histopathologic examination in patients with a history of PCCCL who present with respiratory manifestations, as minute and unexpected findings can directly impact patient care.
Acknowledgements
Financial/nonfinancial disclosures: The authors have reported to Journal of Thoracic Disease that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
References
- Singh HK, Silverman JF, Geisinger KR. Fine-needle aspiration cytomorphology of clear-cell hepatocellular carcinoma. Diagn Cytopathol 1997;17:306-10. [PubMed]
- Kojiro M. Pathology of early liver cancer and similar lesions. 1st ed. Tokyo: Igaku-Shoin Ltd, 1996:35-7.
- Yang SH, Watanabe J, Nakashima O, et al. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int 1996;46:503-9. [PubMed]
- Liu QY, Li HG, Gao M, et al. Primary clear cell carcinoma in the liver: CT and MRI findings. World J Gastroenterol 2011;17:946-52. [PubMed]
- Peng CH, Tang Z, Wu YL, et al. Diagnosis and treatment of five cases of primary clear cell carcinoma of the liver. Chinese Journal of General surgery 2003;10:56-7.
- Li T, Fan J, Qin LX, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol 2011;18:1955-63. [PubMed]
- Lao XM, Zhang YQ, Jin X, et al. Primary clear cell carcinoma of liver--clinicopathologic features and surgical results of 18 cases. Hepatogastroenterology 2006;53:128-32. [PubMed]
- Lai CL, Wu PC, Lam KC, et al. Histologic prognostic indicators in hepatocellular carcinoma. Cancer 1979;44:1677-83. [PubMed]
- Salvucci M, Lemoine A, Saffroy R, et al. Microsatellite instability in European hepatocellular carcinoma. Oncogene 1999;18:181-7. [PubMed]
- Liu Z, Ma W, Li H, et al. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res 2008;38:291-9. [PubMed]
- Ji SP, Li Q, Dong H. Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol 2010;16:764-9. [PubMed]
- Huang NX, Lv Y, Yang SY. Primary hepatic clear cell carcinoma with lung metastasis: a case report. Zhonghua Lin Chuang Yi Shi Za Zhi 2008;2:230-1.
- Edwards C, Carlile A. Clear cell carcinoma of the lung. J Clin Pathol 1985;38:880-5. [PubMed]


