Hyperthermic intrathoracic chemotherapy after extended pleurectomy and decortication for malignant pleura mesothelioma: an observational study on outcome and microcirculatory changes
Introduction
The treatment of malignant pleural mesothelioma represents one of the most important challenges for oncologist, radiotherapists, and particularly surgeons. Surgery should be reserved in selected patients and always as part of a multidisciplinary evaluation and treatment plan (1). The aim of surgery should be to achieve a macroscopic radical resection, associated with a spare of lung parenchyma, in the effort to reduce postoperative complications, improve patient’s quality of life and achieve long term survival (2). In fact it was quite well demonstrated that extrapleural pneumonectomy (EPP) has to be considered only in very selected fit patients with early stage disease, meanwhile pleurectomy and decortication (P/D) represent a valid alternative with comparable survival rates and less functional impact in a wider population of surgical patients (3). The crucial point in P/D is to achieve a complete eradication of mesothelioma cells from the underlying surfaces of lung parenchyma as well as from chest wall, pericardium, and diaphragm (4). In the effort to achieve a complete mesothelioma resection we started an experimental treatment protocol that associates a surgical macroscopic complete resection (MRC) with the Hyperthermic Intra THOracic Chemotherapy (HITHOC) cisplatin based (5). The pharmacokinetics of intrapleural cisplatin perfusion has been widely investigated (6-8) during HITHOC, confirming high platinum concentrations on the chest wall surface with significant cytotoxic effects against microscopic residual disease with minimal systemic toxicity (9). Despite the encouraging survival results of this technique, as reported by Sugarbaker et al. (5) and others European centre (10), our knowledge about the biological impact of the high temperature perfusion on chest cavity is minimal and anecdotal. Recently, in a review about hyperthermic intra-peritoneal chemotherapy (HIPEC), Newton et al. reported a complication rate major than 40%. The study highlights that the most common perioperative complications were respiratory and cardiovascular as well an early unset of kidney failure (11,12). Some authors showed that the intraoperative fluid management played a key role in the onset of postoperative complications after HIPEC (13). Eng et al. (13) demonstrated that an in perioperative fluid rate (IOF) major than 15.7 mL/kg/h reduce significantly the perioperative complications rate in those patients. These studies consider only series of patients who underwent HIPEC for abdominal malignancy and no studies analyse patients treated with HITHOC. In any case the macro and microcirculatory modifications during the procedure as well as in the postoperative days were never described in literature before. Monitoring the early and late microcirculatory changes would add helpful information to improve intra and postoperative patient’s clinical management.
Aim of this study was to describe our experience with HITHOC after pleurectomy and decortication for malignant pleura mesothelioma evaluating the microcirculatory changes with sublingual video-microscopy in the perioperative period in those patients.
Methods
Patients selection and our background
From January 2008 to September 2017 a prospective observational cohort study concerning the multimodal treatment of patients affected by malignant pleural mesothelioma (MPM) was carried on at our Thoracic Surgery Unit. The protocol consisted in: extended pleurectomy and decortication (P/D), associated or not with induction chemotherapy, intraoperative HITHOC with cisplatin, adjuvant chemotherapy based on platinum and pemetrexed (only in patients who do not received induction chemotherapy) and TOMO radiotherapy in all fit patients as last step. All patients received a diagnostic VATS to confirm the diagnosis and were assessed by a whole body CT and PET-CT. Patients were excluded in case of bulky mediastinal involvement, extra-thoracic (abdominal) extension of disease, massive infiltration of the lung or sarcomatoid histology. The pre-operative assessment included: complete pulmonary function tests with static and dynamic volumes, diffusing capacity of carbon monoxide and routine blood gas analysis. The cardio-vascular evaluation concerned in echocardiography with supra aortic trunks assessment. We excluded from surgery patients with a left ventricular ejection fraction lower than 40%, forced expiratory volume in one second lower than 50% of predicted (since only minor lung resection was expected).
The use of hyperthermic endocavitary chemotherapy in oncologic patients was examined and accepted by the local Ethical Committee (Protocol number 620/2007, date 12/10/2007). All patients received an occupational medicine consult to fill in the reports for indemnity if necessary. The diagnosis was reported to the legal authority and to the National register of mesothelioma, as established of the current Italian lows, for each patient.
The HITHOC protocol
The treatment plan concerned patient hydration, for kidney protection, starting 48 hours before surgery and lasting the 4th postoperative day, as shown in Table 1. This setting was borrowed from the normal i.v. cisplatin administration. Surgical procedure, under general anaesthesia with one lung ventilation, was performed via posterolateral thoracotomy at the 6th intercostal space in all patients. Complete pleurectomy and decortication was achieved with sub-segmentary lung resection in case of focal infiltration, the pericardium and diaphragm were carefully freed from the pleura and sutured if torn. In case of grossly infiltration both or only the diaphragm were removed and substituted by a Goretex or PTFE mash. After an adequate haemostasis and control of air leak (either by hands sutures and application of sealant devices), 3 pleural drainages and a temperature probe were positioned into the chest cavity (axillary, posterior laying on the diaphragm and anterior to the apex) and the chest wall closed. The patient remain in lateral position with the lung deflated, the Performer LRT System (Medtronic LTD) is connected to the drainage and starts to fill the pleural cavity with the primer physiologic solution reaching the desired temperature of 42 °C. Reached the temperature the perfusionist starts the infusion of cisplatin in the primer solution that will run for 60 minutes at about 42 °C. Usually we start with 2,000 mL of primer reaching between 2,500 and 3,000 mL depending of patient’s half real TLC. The dose of cisplatin is increased during the study; according to our consultant oncologist we started with 80 mg/m2 but after the first cases we reached a dosage of 180 mg/m2. At the end of procedure the chest cavity is perfused with a saline solution for 10 minutes in the effort to wash out the residual cisplatin and then lung is inflated. All patients were transferred in the ICU for the first 12–24 h. After discharge, patients who did not received induction chemotherapy before surgery were sent to the medical oncology team for adjuvant chemotherapy (pemetrexed 500 mg/m2 + cisplatin 75 mg/m2 every 21st day for 4 to 6 cycles). All patients who had received induction chemotherapy and those considered suitable after adjuvant chemotherapy were sent to the radiation therapy.

Full table
Haemodynamics and microcirculation evaluation
After 4 years from the beginning of the study, a prospective and observational sub-study concerning 10 consecutive patients undergoing extended P/D followed by HITHOC was started. These patients underwent sublingual microcirculatory monitoring, which was adopted as a routine procedure since 2012. The local Ethical Committee approved this prospective study, and all patients were informed and gave written informed consent the day before surgery (Protocol CEL_AOUS13/11/2012, date 23/11/2012). The patients enrolled in the study were firstly admitted at the Thoracic Surgery ward and after surgery were admitted to the Intensive Care unit (ICU).
Haemodynamic parameters were collected at eight consecutive times: the day before the surgery (T1), after the induction of anaesthesia (T2), at the end of the surgical phase just before the HITHOC beginning (T3), 5 and 30 minutes after the beginning of HITHOC (T4 and T5, respectively), 5 minutes from the end of the HITHOC (T6), after the admission to the ICU and stabilization of haemodynamic and ventilatory parameters (T7), and at the discharge from the ICU (T8). Cardiac output (CO) was calculated with MostCare (14). Systemic vascular resistance (SVR), oxygen delivery (DO2), and oxygen extraction rate (O2ER) were calculated using standard formulas. Arterial blood pressure and central venous pressure (CVP) were obtained with standard arterial and venous catheters; the latter served to measure central oxygen venous saturation (ScvO2).
At the same times we assessed the sublingual microcirculation with Sidestream Dark Field technique (SDF, MicroVision Medical, Amsterdam, The Netherlands). Saliva was removed with gauzes and the SDF probe was positioned at the sublingual mucosa (Figure 1).
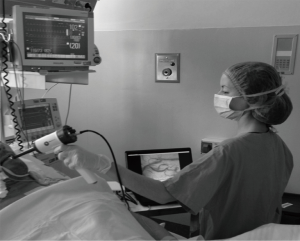
Microcirculatory analysis
The SDF system has been extensively described elsewhere (15). Briefly, in SDF, imaging illumination is provided by surrounding a central light guide by concentrically placed light emitting diodes (LEDs). Light from the SDF probe penetrates the tissue and illuminates the tissue-embedded microcirculation by scattering. The LEDs emit at a central wavelength to ensure optimal optical absorption by haemoglobin in RBCs independently of its oxygenation state. In the final image, RBCs are visualized as dark moving globules against a white/grayish background. To improve imaging of flowing RBCs the LEDs provide pulsed illumination (15,16). We recorded five consecutive videos of 20 seconds each at different areas of the sublingual mucosa. Thereafter, video sequences of the microcirculation were analysed offline by two investigators (A.D. and F.F.) blinded to clinical data (17). Using a cut-off value of 20 µm in diameter, the vessels were separated according to their size into large and small. We used two scores to assess microcirculatory parameters. The score (18) is based on the principle that vessel density is proportional to the number of vessels crossing arbitrary lines. For each subject we calculated total vascular density (TVD, vessels mm−2) and proportion of perfused small vessels (PPV, %), which physiologically represent the number of micro-vessels whose blood flow capability to tissue is adequate. TVD was calculated as previously described (17). To calculate PPV, we visually categorized blood flow in the micro vessels as present, absent, or intermittent (18). We computed the proportion of perfused vessels (total, large, and small) as the number of vessels continuously perfused during the 20 seconds observation period divided by the total number of vessels of the same type. For each subject, data from five areas of sublingual mucosa were averaged. The second score was the microvascular flow index (MFI, arbitrary units), which represents the predominant type of micro-vascular blood flow in a specific mucosal region. The score ranged from 0 to 3, describing a flow as absent (0 points), intermittent (1 point), sluggish (2 points), or normal (3 points) (19). Physiologically, the higher the MFI the better the microvascular blood flow and perfusion (20). Finally, we calculated the heterogeneity index (HI, %), which represents the heterogeneity of blood flow between different sublingual mucosal areas. A low HI indicates an adequate tissue distribution of micro-vessels whose blood flow is normal. Physiologically, higher is the HI and lower is the microcirculatory function (20).
Statistical analysis
Data are presented as number (percentage), mean (SD), or median (IQR), and total range. The χ2 test or Fisher exact tests were used for binary variables. Normal distribution of data was assessed with the Kolmogorov-Smirnov test. For continuous data, paired Student’s t-test or Wilcoxon-test, when appropriate, was used. Repeated measures analysis of variance (ANOVA) was applied to microcirculatory and haemodynamic data to evaluate within-subjects (time) effects. The Bonferroni correction was used to account for multiple comparisons. The Pearson’s linear regression test, or Spearman’s correlation, when appropriate, was applied to test correlation between variables. Statistical significance was considered for P<0.05. The statistical analysis was performed with dedicated software (IBM SPSS Statistics, Version 20.0. Armonk, NY: IBM Corp.; GraphPad Prism 5, San Diego, CA, USA).
Results
Clinical results of HITHOC
Forty-one patients were enrolled in the HITHOC protocol (33 males, 8 females); mean age was 64±6 years (range, 76–54 years); affected side was right in 22 patients and left in 19. Thirty-seven patients presented a history of asbestos exposure, both related to direct or parental working experience or environmental contamination. The mean cisplatinum dose was 145±33 mg/m2 (range, 80–180 mg/m2); perfusion time was 58±5 minutes (range, 40–64 minutes) and average temperature was 41.4±0.5 °C (range, 40.1–42.5 °C). We found a median survival of 25 months with significant improvement in survival in the patients without residual disease (32 vs. 17 months, P=0.044). Moreover, we found that in patients who received a cisplatinum dose higher than 100 mg/m2 had better median survival (31 vs. 18 months, P=0.020) than those who underwent cisplatinum treatment with a dose lower than 100 mg/m2.
Hemodynamic and microcirculatory data
Hemodynamic and microcirculatory data were collected in 10 patients, 8 male and 2 females (mean age 68.6±9.0, and body surface area of 1.9±0.1 m2). All patients had arterial hypertension, and one patient had diabetes.
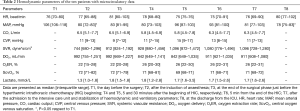
Hemodynamic data are reported in Table 2. Mean arterial pressure (MAP) significantly decreased at T2, with respect to T1 (P=0.05; Table 2). CO, CVP, DO2, O2ER, and ScvO2, did not change significantly over the time (Table 2). All patients needed infusion of noradrenalin from T4 to T6. TVD significantly decreased from T1 to T3, T5, and T8. Similarly, PVD significantly decreased from T1 to T3 and T8, and MFI from T1 to T6 and T8 (Table 3). PPV and HI did not change over the study period. No correlation was found between hemodynamic parameters (MAP, CO, CVP, DO2, O2ER, ScvO2) and microcirculatory data (TVD, PVD, PPV, MFI, HI), at any time of the study (data not shown).
Full table

Full table
Discussion
Our study confirms that pleurectomy and decortication combined with HITHOC is associated with a significant improvement median survival in those patients respect to the no treated patients. However, the use of HITHOC has a significant impact in the microcirculatory parameters during and after the procedure. These changes were independent from the global hemodynamic variables and this could explain the difficult postoperative manage and the incidence of cardiocirculatory complications (15).
In our series the association of P/D and HITHOC with chemotherapy and, in suitable patients, radiotherapy leads to an elevated median 5 years survival, particularly significant in those who received a radical resection. These results are pushing us to improve the HITHOC experience. However, to achieve a radical macroscopic resection we need a precise e meticulous inspection of chest wall and lung surface and partial or complete diaphragmatic resection, often associated also with pericardial resection and reconstruction.
In this setting, the surgical stress for the patient could be detrimental when associated with HITHOC. For this reason it is mandatory an appropriate anaesthetic protocol associated with advanced clinical monitoring to guide and improve intraoperative and postoperative patient’s management. Our study showed a complete dichotomy between the haemodynamic parameters and microcirculatory changes. The HITHOC induced a peripheral vasodilatation with consequent systemic pressure drop. This phenomenon can be easily managed with noradrenalin administration. Opposite, the changes in microcirculation were evident for a long time, as they were still present at discharge from ICU. There were some considerations about the different trends of hemodynamic and microcirculatory variables in these patients. Several authors demonstrated that macro- and micro-dynamic are not correlated either in septic patients or in patients undergoing cardiac surgery (21-24). Also, it has been demonstrated that an increase in MAP with norepinephrine infusion may not change microcirculatory function (20). In the present study, MAP did not change during HITHOC because of early infusion of noradrenalin to prevent excessive hyperthermia-induced vasodilation.
An inflammatory response syndrome induced by the surgery might also induce changes in microcirculation, as showed in patients undergoing cardiac surgery with or without extracorporeal circulation (24). However, also the general anaesthesia may contribute to the changes on microvascular perfusion, even though its effects are transient (24).
Finally, the elevated temperature used during HITHOC (cisplatin at about 42 °C) would have affected microcirculatory parameters. In fact, it has been demonstrated that hypothermia change microvascular density and flow in the early post-resuscitation phase after cardiac arrest (25). We could hypothesize that the high body temperature impairs microvascular perfusion, as our findings showed, inducing a reduction of both number of microvessels and microflow index. Our hydratation protocol was borrowed from the protocols commonly used in patients who receive systemic chemotherapy with cisplatin, in that setting the fluid load and diuretics should prevent a possible renal failure related to cisplatin toxicity. On the contrary, in patients who receive HITHOC the fluid load can also reduce the microvascular impairment restoring the normal tissue perfusion. This process takes days but is most evident in the first 72 h. The use of colloid and blood transfusion is much more effective in restoring microcirculation and reducing tissue damaging. In our series we observed that more than 50% of patients needed a blood transfusion after the first 48 and 72 h for a drop in haemoglobin under 8 g/dL. This seems to be the cause of the partial resolution of the microvascular impairment with an improvement in the distribution volume. In conclusion, the study of microcirculation changes during the HITHOC procedure provided us some suggestions on how to change the protocol care. Practically, the days of fluid load could be reduced (e.g., from 5 to 3) and desamethason in the postoperative could be reduced or stopped, as no evident advantage was observed with the use of if in preventing microvascular impairment due to systemic inflammatory reaction. Moreover, the practice of blood transfusion could be introduced earlier postoperatively to improve and accelerate the recovery of microcirculatory function.
Our study has several limitations. Firstly, only ten patients were monitored using SDF; however it was adequate to demonstrate the changes in haemodynamic and microvascular parameters in the perioperative period. Secondarily, our study protocol did not include data on tissue oxygenation (e.g., near-infrared spectroscopy), and information on muscle microvascular reactivity cannot be provided. Finally, we did not collect data regarding the fluid therapy (amount and type) and could not evaluate its association with microcirculatory changes. It is well known that hemodilution and hypovolemia could impair microvascular perfusion and tissue oxygenation (26). In addition, crystalloids and colloids may affect differently the microcirculatory function (27). In this view, SDF monitoring could be useful to assess the effects of fluid therapy during HITHOC, in order to optimize microcirculatory and global haemodynamic variables.
In conclusion, HITHOC after pleurectomy and decortication seems a useful strategy to improve the outcome of the patients affected by malignant pleural mesothelioma. Hyperthermic intrathoracic chemotherapy affects the microcirculation, which can be easily assessed by sublingual video-microscopy in the whole perioperative period.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The use of hyperthermic endocavitary chemotherapy in oncologic patients was examined and accepted by the local Ethical Committee (Protocol number 620/2007, date 12/10/2007). All patients received an occupational medicine consult to fill in the reports for indemnity if necessary. The diagnosis was reported to the legal authority and to the National register of mesothelioma, as established of the current Italian lows, for each patient.
References
- Saddoughi SA, Abdelsattar ZM, Blackmon SH. National Trends in the Epidemiology of Malignant Pleural Mesothelioma: A National Cancer Data Base Study. Ann Thorac Surg 2018;105:432-7. [Crossref] [PubMed]
- Waller DA. Lung-sparing total pleurectomy: the surgical option of choice in malignant pleural mesothelioma? Eur J Cardiothorac Surg 2013;44:123-4. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pelural pneumoectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. The management of the diaphragm during radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2016;50:311-6. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress; September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Rusch VW, Niedzwiecki D, Tao Y, et al. Intrapleural cisplatin and mitomycin for malignant mesotelioma following pleurectomy: pharmacokinetic studies. J Clin Oncol 1992;10:1001-6. [Crossref] [PubMed]
- Yasumoto K, Shimokawa T, Nagashima A, et al. Pharmacokinetics of cisplatin instilled into the pleural cavity following panpleuropneumonectomy in patients with malignant pleurisy due to lung cancer. J Surg Oncol 1993;54:67-70. [Crossref] [PubMed]
- Ratto GB, Civalleri D, Esposito M, et al. Pleural space perfusion with cisplatin in the multimodality treatment of malignant mesotelioma: a feasibility and pharmacokinetic study. J Thorac Cardiovasc Surg 1999;117:759-65. [Crossref] [PubMed]
- Ambrogi MC, Bertoglio P, Aprile V, et al. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 2017 pii: S0022-5223(17)32399-1.
- Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol 2016;7:99-111. [PubMed]
- Kerscher C, Ried M, Hofmann HS, et al. Anaesthetic management of cytoreductive surgery followed by hyperthermic intrathoracic chemotherapy perfusion. J Cardiothorac Surg 2014;9:125. [Crossref] [PubMed]
- Eng OS, Dumitra S, O'Leary M, et al. Association of Fluid Administration With Morbidity in Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. JAMA Surg 2017;152:1156-60. [Crossref] [PubMed]
- Romagnoli S, Franchi F, Ricci Z, et al. The Pressure Recording Analytical Method (PRAM): Technical Concepts and Literature Review. J Cardiothorac Vasc Anesth 2017;31:1460-70. [Crossref] [PubMed]
- De Backer D, Ospina-Tascon G, Salgado D, et al. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Med 2010;36:1813-25. [Crossref] [PubMed]
- Ince C. Sidestream dark field (SDF) imaging: an improved technique to observe sublingual microcirculation. Crit Care 2005;9:72. [Crossref]
- Scolletta S, Marianello D, Isgrò G, et al. Microcirculatory changes in children undergoing cardiac surgery: a prospective observational study. Br J Anaesth 2016;117:206-13. [Crossref] [PubMed]
- De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care 2007;11:R101. [Crossref] [PubMed]
- Lamia B, Chemla D, Richard C, et al. Clinical review: interpretation of arterial pressure wave in shock states. Crit Care 2005;9:601-6. [Crossref] [PubMed]
- Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 2007;49:88-98, 98.e1-2.
- Jhanji S, Stirling S, Patel N, et al. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med 2009;37:1961-6. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009;13:R92. [Crossref] [PubMed]
- De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg 2009;88:1396-403. [Crossref] [PubMed]
- Donadello K, Favory R, Salgado-Ribeiro D, et al. Sublingual and muscular microcirculatory alterations after cardiac arrest: A pilot study. Resuscitation 2011;82:690-5. [Crossref] [PubMed]
- Funk W, Baldinger V. Microcirculatory perfusion during volume therapy. A comparative study using crystalloid or colloid in awake animals. Anesthesiology 1995;82:975-82. [Crossref] [PubMed]
- Küpper S, Mees ST, Gassmann P, et al. Hydroxyethyl starch normalizes platelet and leukocyte adhesion within pulmonary microcirculation during LPS-induced endotoxemia. Shock 2007;28:300-8. [Crossref] [PubMed]
- van Bommel J, Siegemund M, Henny CP, et al. Microvascular shunting in severe normovolemic hemodilution. Anesthesiology 2001;94:152-60. [Crossref] [PubMed]


