Diagnostic and therapeutic challenges in managing persistent air leaks
Introduction
Persistent air leak (PAL) secondary to a medical condition is defined as an alveolar-pleural fistula (APF) that persists for more than 5 days. Patients with PAL are usually managed surgically for leak repair and pleurodesis (1). In patients who fail or are deemed poor surgical candidates, a non-surgical intervention is usually attempted. Such non-surgical interventions consist of chemical pleurodesis and other anecdotal pleural and bronchoscopic approaches (2). The Food and Drug Administration (FDA) has approved the placement of intrabronchial valves (IBV) for the management of PAL under its Humanitarian Device Exemption (1). Many case series and retrospective studies have reported high success rates with these anecdotal techniques, but currently there is a lack of robust comparative data. The paucity of prospective studies to evaluate their efficacy and safety has made the choice of the optimal non-surgical approach controversial and operator-dependent.
In this report, we present three cases of medical PAL that exemplify the diagnostic and therapeutic challenges inherent in the management of this condition and illustrate how it can be treated with a combination of surgical and non-surgical approaches.
Case presentation
Case 1
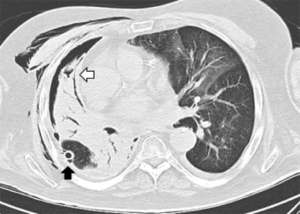
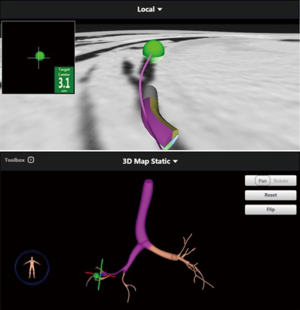
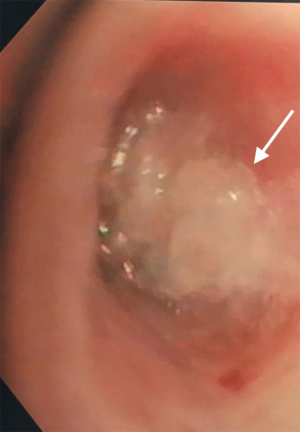
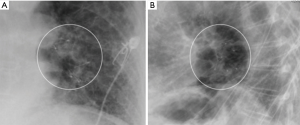
A 68-year-old man former smoker with myasthenia gravis on chronic corticosteroids and with stage IIIA non-small cell lung carcinoma (NSCLC) had been managed with chemotherapy and radiation therapy. He presented with acute dyspnea due to spontaneous right tension pneumothorax requiring urgent chest tube placement. Chest computed tomography (CT) showed almost complete right lung opacification consistent with radiation pneumonitis. His hospital course was complicated by PAL and pneumonia, and the patient subsequently underwent right video-assisted thoracoscopic surgery (VATS). What appeared to be a right middle lobe (RML) APF was identified. A surgical sealant (Progel, Bard Davol, Warwick, RI, USA) and talc were applied, and a chest tube was left in place. Following surgery, the patient continued to have PAL and was transferred to our institution for further management. A repeat chest CT revealed a lung defect at the level of the medial segment of the RML (Figure 1). During bronchoscopy, a balloon-tipped catheter inflated in the bronchus intermedius (BI) effectively stopped the air leak as indicated by cessation of bubbling in the pleural drainage system after several respiratory cycles. This was followed by RML bronchial and segmental occlusion, totally abolishing the air leak upon occlusion of the medial segment. Three therapeutic bronchoscopies were performed on separate days. Navigational bronchoscopy (SuperDimension, Minnesota, MN, USA) was used in the first procedure to guide fibrin glue instillation distally at the level of the identified APF using the Edge™ 45° extended working channel (EWC) (Figure 2). The EWC was then retracted proximally as three milliliters of a fibrin sealant (Evicel ®Ethicon, Somerville, NJ, USA) were injected to fill up the bronchus of the medial segment of the RML (Figure 3). The air leak diminished significantly but recurred a few hours later. Forty-eight hours after the first bronchoscopy, the patient underwent a second procedure during which we used a cellulose polymer (Surgicel®, Ethicon, Somerville, NJ, USA) to pack the airway. This was followed by immediate instillation of a surgical sealant (CoSeal®, Baxter, Deerfield, IL, USA) using a radial endobronchial ultrasound guide sheath (K-203, SG-201C, 2.55 mm; Olympus America) as its tip was easier to adapt to the hub of the CoSeal® syringe. This combination controlled the air leak for only less than 24 hours. At this point, we decided to proceed with placement of an IBV in the RML bronchus. It was felt that occluding the entire RML bronchus would not have a significant impact on the patient’s respiratory status given the extent of post-radiation damage to that lobe. The air leak significantly decreased, then resolved, and the chest tube was removed 2 days later. The patient was subsequently discharged to an acute rehabilitation facility. Unfortunately, a month later he was readmitted with sepsis and a pleuro-cutaneous fistula with air leak at the site of the previous chest tube insertion. A chest tube was placed followed by an Eloesser window procedure to drain the infected pleural space. The patient remained hospitalized for about 2 months and expired from septic and cardiac complications.
Case 2
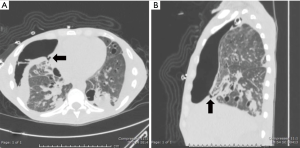
A 34-year-old man was transferred to our institution for severe acute respiratory distress syndrome (ARDS) secondary to influenza B infection. The patient was started on extracorporeal membrane oxygenation (ECMO), but he developed a large right pneumothorax secondary to barotrauma from mechanical ventilation, for which a large-bore chest tube was placed. On hospital day 9, the patient developed a left pneumothorax and had a left chest tube placed. The left pneumothorax resolved, allowing chest tube removal 21 days after placement. The patient continued to have PAL from the right chest tube. On hospital day 33, a surgical adhesive—a combination of bovine serum albumin and glutaraldehyde (BioGlue, Cryolife Inc. GA, USA)—was instilled via the right chest tube; however, the air leak persisted. Since the patient was deemed a poor surgical candidate, he was evaluated for bronchoscopic management of PAL. The site of presumed BPF was identified on chest CT (Figure 4), which also showed extensive fibrocystic degeneration of the right lung as a complication of ARDS. At bronchoscopy, the balloon occlusion technique identified the medial segmental bronchus of the RML as the culprit airway, and three IBVs (Spiration®, Olympus Respiratory, Redmond, WA, USA) were placed in three different sub-segmental bronchi. The air leak temporarily stopped but then reappeared, albeit at a slower rate. The decision was made to perform a repeat bronchoscopy and re-evaluate the lateral segmental bronchus of the RML. Four days later (day 46 of PAL) during the second bronchoscopy, another valve was placed in the lateral segment of the RML. Complete resolution of the air leak was achieved. The right chest tube was removed 2 days later.
Case 3
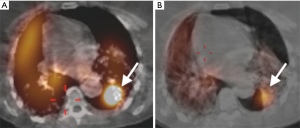
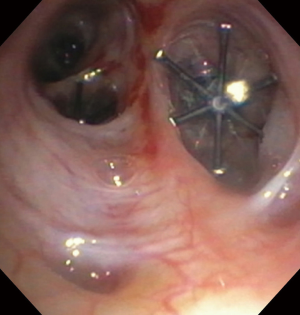
A 57-year-old woman with a past medical history of idiopathic pulmonary fibrosis (IPF) and chronic hypoxic respiratory failure that was initially treated with pirfenidone and later with nintedanib was admitted to the hospital for a left secondary spontaneous pneumothorax (SSP). She developed PAL that was initially managed with VATS bullectomy and talc pleurodesis. She was transferred to our institution, where she underwent doxycycline pleurodesis that did not abolish the PAL. This was followed by instillation of a fibrin sealant through the pleural drain, but the air leak persisted. The air leak was too small and intermittent for bronchoscopic localization by the balloon occlusion method. Therefore, 99mTc-tagged diethylenetriamine penta-acetate single photon emission computed tomography scanning (DTPA-SPECT-CT) was performed and identified the air leak, suggesting a left lower lobe (LLL) superior segment location (Figure 5). She was discharged home with the pleural drain connected to a Heimlich valve. The patient continued to have PAL two months later and the decision was made to place IBVs, which would be a reversible therapeutic option should the patient develop adverse effects such as hypoxia. She underwent bronchoscopy with insertion of two valves (Spiration®, Olympus Respiratory, Redmond, WA, USA) in the superior segmental bronchus of the LLL. Culprit airway localization was based on the SPECT-CT fusion scan findings rather than results of balloon occlusion testing because of the minimal and intermittent nature of air leak as mentioned previously. The air leak appeared to have resolved in the immediate post-procedure period but then reappeared a few hours later and was larger in size. At repeat bronchoscopy, balloon occlusion was performed since the degree of air leak had now increased, and it was found that all segments of the LLL contributed to the leak. A total of four additional Spiration® valves were placed in the anteromedial basal segment bronchus, lateral basal segment bronchus, and posterior basal segment bronchus (Figures 6,7). The air leak resolved and the pleural drain was removed three days later. The patient was discharged home the following day.
Discussion
Many patients with PAL are suboptimal or prohibitive operative candidates. In recent years, multiple bronchoscopic modalities have emerged as options for PAL management (2). Of these options, it is specifically worth mentioning the IBV, originally designed for bronchoscopic lung volume reduction in emphysema patients. These valves are now FDA-approved under a humanitarian device exemption for PAL occurring post-lobectomy, segmentectomy and lung volume reduction surgery. Many authors have demonstrated the utility of IBV placement and other anecdotal approaches for non-operative BPF closure (3-6). Here, we have presented a case series of three patients with PAL who were challenging to manage using the combined bronchoscopic, pleural, and surgical approaches.
The first case was complicated by multiple factors that impair the healing process of the APF. These include long-term corticosteroid use, recent chemotherapy, radiation pneumonitis, pneumonia, and pleural soiling. The patient had emphysema, which is an additional aggravating factor.
The second case represents classic barotrauma from injurious mechanical ventilation in severe ARDS, again emphasizing the poor surgical candidacy of many PAL patients. Pleural management using fibrin glue through the chest tube was based on case reports. It was unlikely to work given the ongoing positive pressure mechanical ventilation, visible alveolar-pleural defect as well as fibrocystic lung destruction on chest CT. For similar reasons, chemical pleurodesis was unlikely to work and IBV placement was chosen as the next step.
The third case illustrates multiple challenges both in localizing and treating the APF. That patient’s PAL had persisted for over 6 weeks. Despite a long-term outpatient pleural catheter that was attached to a Heimlich valve, the air leak remained albeit; small and intermittent. Therefore, it was considered improbable to successfully identify the airway leading to the APF using the balloon occlusion method during bronchoscopy. Other reported methods such as oxygen insufflation of airway segments to increase the air leak flow and improve the chance of APF identification were not attempted since the patient was in chronic respiratory failure secondary to IPF and there was a concern of worsening the APF, which might lead to clinical deterioration and the inability to successfully manage the larger air leak (7). 99mTc-tagged DTPA-SPECT CT fusion imaging has been reported to successfully localize air leaks, but data are limited to case reports (6). In the case published by Ceulemans et al., a patient presented with SSP, and the PAL was treated surgically without any success. SPECT imaging accurately identified the site of leak in that case. In our patient, the SPECT fusion scan suggested a leak at the level of the LLL superior segment, which was the first step in identifying the site of the APF. Valve placement in the sub-segments of the LLL superior segment did not stop the leak. This could have been due to the patient’s severe interstitial lung disease resulting in significant bronchoalveolar destruction and therefore collateral ventilation connecting the basal segments of the LLL to the superior segment. This would explain the need to insert valves into all segments of the LLL to completely abolish the air leak. For this patient, we did not consider the use of sealant for occlusion due to her high oxygen requirement and difficulty of removing the glue plug in case of significant complications such as worsening hypoxia, hypercapnia, and atelectasis. Valve placement successfully treated PAL in two patients (cases 2 and 3). In the first case, the patient expired from septic complications despite using surgical, pleural, and bronchoscopic approaches. In all cases, the bronchoscopic intervention took place late in the course of PAL management.
From the anesthesia standpoint, PALs are challenging to manage. If a bronchopleural fistula is present in a ventilated patient, there is a loss of delivered tidal volume, persistent lung collapse, and inability to apply PEEP. In mechanically ventilated patients the goal is to maintain adequate ventilation and oxygenation while reducing the fistula flow to allow the leak to heal. Other modes of ventilation include high-frequency ventilation, oscillation and differential lung ventilation through a double lumen tube (8,9).
Conclusions
The management of PALs is commonly challenging. Due to the lack of comparative data among the different pleural and bronchoscopic techniques, the preferred approach is based on operator experience and local availability.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This is a case-based report. It’s unneccessary to obtain Informed Consent since no patient can be identified based on the information in this report.
References
- Lazarus DR, Casal RF. Persistent air leaks: a review with an emphasis on bronchoscopic management. J Thorac Dis 2017;9:4660-70. [Crossref] [PubMed]
- Keshishyan S, Revelo AE, Epelbaum O. Bronchoscopic management of prolonged air leak. J Thorac Dis 2017;9:S1034-S1046. [Crossref] [PubMed]
- Snell GI, Holsworth L, Fowler S, et al. Occlusion of a broncho-cutaneous fistula with endobronchial one-way valves. Ann Thorac Surg 2005;80:1930-2. [Crossref] [PubMed]
- Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg 2011;91:270-3. [Crossref] [PubMed]
- Gaspard D, Bartter T, Boujaoude Z, et al. Endobronchial valves for bronchopleural fistula: pitfalls and principles. Ther Adv Respir Dis 2017;11:3-8. [Crossref] [PubMed]
- Travaline JM, McKenna RJ, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- Vial MR, Lan C, Cornwell L, et al. Endobronchial oxygen insufflation: a novel technique for localization of occult bronchopleural fistulas. Ann Am Thorac Soc 2013;10:157-9. [Crossref] [PubMed]
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Ross IB, Fleiszer DM, Brown RA. Localized tension pneumothorax in patients with adult respiratory distress syndrome. Can J Surg 1994;37:415-9. [PubMed]


