Insomnia and cognitive behavioural therapy—how to assess your patient and why it should be a standard part of care
Introduction
Normal sleep relies upon two distinct but overlapping neuronal circuits. The homeostat drives an increasing pressure to sleep after every hour awake and the circadian rhythm drives alertness in the day and sleep at night with light intensity as the strongest external timekeeper (1). Both total sleep time and the circadian rhythm change over the course of our lives and tend to fragment and weaken over time (2). Teenagers and young adults need 8–9 hours of sleep on average with a delay in sleep phase such that many fall asleep after rather than before midnight (3). There is phase advance (i.e., falling asleep earlier) with every decade that passes with adults falling asleep by 30 minutes earlier a decade on average from the third decade onwards. There is increasing sleep fragmentation and increased time to fall asleep in otherwise healthy older adults with and without sleep complaints (4).
In modern life, we manipulate our light levels to disrupt and confuse our circadian rhythm. First the industrial revolution and then, more recently, the digital revolution have led to shift work and sleep restriction as a widespread phenomenon across society. At least 30% of US adults sleep less than 6 hours a night on a regular basis (5). This may be at least part of the reason that insomnia and other sleep disorders remain under diagnosed and undertreated (6). In addition there may be a tendency to attribute insomnia in those with mental or physical health problems to an existing diagnosis, rather than consider insomnia as a distinct and treatable disorder.
What is insomnia disorder and why does it develop?
Simply put, insomnia is difficulty falling asleep and difficulty staying asleep with impact on daily function which is not due to a disruptive sleeping environment or another sleep disorder.
Insomnia disorder remains the commonest sleep disorder in primary care with multiple cohort studies estimating at least 5–10% of the population affected (7-9). The diagnostic criteria have been simplified and insomnia disorder is now a diagnostic entity in its own right in the most recent ICSD3 and DSM5 classifications (10,11). The current criteria emphasize impact on a variety of daytime functions, difficulty for at least 3 or more nights a week and for more than 3 months (Table 1 for DSM5 diagnostic criteria).

Full table
Increasing age is a risk factor due in part to the natural tendency for a more fragmented night and decreasing melatonin output. It is more common in women and it is commonly comorbid with other mental and physical health problems (12). At least 40% will have anxiety disorder. (Insomnia disorder is now called comorbid insomnia if it is associated with another physical or mental health problem). Persistent insomnia remains the single biggest risk factor for depression (13).
Acute stressors are a common cause of acute insomnia, for example an argument at work or a systemic illness. This usually resolves when the stressor is removed (14). However for chronic insomnia to develop, there usually have to be perpetuating factors. One of the most widely accepted models for chronic insomnia is based on the Spielman three ‘P’s model (15) with predisposing factors (certain personality traits), a precipitating trigger (the acute stressor) but then perpetuating or maladaptive behaviours that worsen sleep and disrupt both the normal homeostatic and circadian drivers to sleep. The typical example is people who nap during the day and disrupt the natural drive to night sleep.
A hypervigilant phenotype is typical with a “racing mind” that finds it hard to switch off. “As soon as I climb the stairs, the lights go on in my head” are typical statements heard in clinic. An increased effort around sleep is often part of the description. Espie and others have emphasized the increased attention to sleep and effort to control sleep that paradoxically increases rather than decreases alertness in the bedroom (16). Hyperarousal develops such that those with insomnia have a fragmented nocturnal electroencephalogram (EEG) with microarousals, increased heart rate and blood pressure relative to normal sleepers (17). Increased worry and rumination around sleep often maintain the problem (16). All of this is a combination of factors that leads to “immoderate watchfulness”.
How to diagnose insomnia and common mimics
For many the diagnosis can be made with a careful history alone (a suggested sleep history with necessary screening questions is listed in Table 2) alongside patient completed sleep diaries over at least 1 week. For many the most helpful question is to ask the patient to talk through a typical 24 hours; the description of being fatigued but awake during the day with frustrated long gaps awake at night is characteristic. It is usually distinct to those with, for example, sleep apnoea, which may have a fragmented night but usually can’t maintain daytime wakefulness and gaps awake at night are much briefer.

Full table
A systemic examination is required (but rarely helpful—cases of nocturnal paroxysmal atrial fibrillation or hyperthyroidism that has been missed or presents with insomnia alone are uncommon). Review of the prescription list for all medications that can be stimulant is important (for example evening inhalers, adrenergic nasal sprays) and an assessment of mood is also needed given the common comorbidity of affective disorder. If daytime symptoms of depression or anxiety dominate then this needs to be addressed.
Patients complain about the impact of poor sleep upon work and driving but there is often a mismatch between perceived performance and more objective assessments of for example car crashes or failure at work. This is different to the sleep apnoea history where driving and daytime function is usually affected (18). The prior and current use of sleeping tablets including over the counter preparations needs to be documented as these can be contributing to some of the daytime fatigue.
There are a number of insomnia mimics and patients may have more than one sleep disorder so further investigations may be needed. Polysomnography is not required for the diagnosis unless another sleep disorder is suspected and actigraphy is rarely used outside of research. If done, polysomnography typically shows a fragmented night with reduced sleep efficiency, a prolonged sleep latency and short rapid eye movement (REM) latency (17). There is often an element of sleep misperception with patients estimating less time asleep in the lab than is objectively measured. However, explaining this rarely helps the patient’s perception of a poor night.
Common insomnia mimics (particularly in those without a bed partner) include restless legs syndrome (RLS) and periodic limb movement disorder, obstructive sleep apnoea (OSA) where patients may perceive night awakenings, a circadian rhythm disorder and in particular delayed sleep phase syndrome in younger patients where the key problem is difficulty initiating but not usually maintaining sleep. There are a small number of truly short sleepers who awake refreshed after just 5 hours but feel this is not enough compared to societal norms but for insomnia disorder there has to be associated daytime functional impairment.
Those with daytime sleepiness should prompt screening for secondary causes of insomnia.
At least 10% of those seen in our regional sleep service have RLS (19) as the cause for a fragmented night. A highly specific question to screen is “when you try to relax in the evening or sleep at night, do you ever have unpleasant, restless feelings in your legs that can be relieved by walking or movement?” (20). RLS can be episodic but should be treated if symptomatic before insomnia-specific cognitive behavioural therapy (CBTi) regimes are started.
Twenty percent of those with OSA perceive a restless and unrefreshing night although the other clues of snoring, dry throat and daytime sleepiness should prompt further investigations. Gastro-oesophageal reflux can be surprisingly toxic to sleep as another secondary cause. It can be hard to distinguish insomnia alone from insomnia with comorbid OSA in those over 65 (21) who can often have atypical presentations with lower body mass index (BMI). Therefore, there should be a lower threshold for screening for OSA in older patients with a respiratory sleep study with any daytime sleepiness or snore as it will affect treatment. The clue is often falling asleep easily but waking in the second half of the night.
The other common mimic in younger patients is the circadian rhythm disorder of delayed sleep phase syndrome where patients can’t initiate sleep but then stay asleep well. Sleep diaries and an accurate history can usually distinguish the two. Delayed sleep phase syndrome is more often a persistent problem from late childhood, in particular males (22,23), For many the key question is “if you were allowed to sleep when you wanted, would you sleep well?”
Shift work can produce shift work sleep disorder. At least 20% of the western world carry out shift work and, for at least 20%, this significantly disrupts sleep. Shift work decreases total sleep time and disrupts circadian rhythm with substantial evidence for increased mortality, morbidity and metabolic dysregulation compared to non-shift workers (24). Groups at increased risk include; women, those over 40 and those working rotating or back to back night to day shifts (25). However, CBTi is not appropriate for those who remain on a variable schedule due to the very nature of the therapy which focuses upon a fixed pattern of bed and wake time.
The sleep diary—necessary for diagnosis and therapy
The cornerstone of both diagnosis and subsequent therapy is sleep diaries where a typical pattern of far more time in bed than asleep is usually seen.
One of the most simple and cost effective diagnostic tests available to all wherever they work is asking the patient to keep a sleep diary for at least 1 week, ideally 2. This may be the only diagnostic test needed for insomnia disorder; it can also distinguish between insomnia and circadian rhythm disorder such as delayed sleep phase syndrome. There are a number available as online or paper records but as a minimum this needs to include; lights out, time in bed and estimated time asleep, any daytime napping and ideally lifestyle factors such as caffeine intake, alcohol, meals and exercise. There is debate about the length of time needed for diagnosis but the recent ICSD3 highlights that certain circadian rhythm disorders will be missed with a single week of diaries (10). For all of us, weekends are different to weekdays even in those who are not employed or work shifts.
Some diaries ask patients to rate satisfaction with sleep on a nightly basis but the author does not do this—generally if they were satisfied with sleep they wouldn’t be attending a sleep clinic or completing a diary!
One key instruction is to complete the diary once a day and not in the middle of the night. Increasing numbers of patients wear and look at data from commercial accelerometers including fitbits, jawbones and smart phones. However these devices overestimate movement as wake (26) so should not be used as a substitute for the diary. They can in themselves exacerbate insomnia. If patients awake refreshed and do not perceive a problem falling asleep then they should largely avoid looking at movement based data. Some typical diaries are shown from normal and abnormal sleepers (Figure 1).
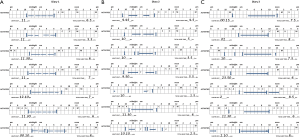
The sleep efficiency (total sleep time/time in bed) over 7 or 14 days can then be calculated. Normal sleep is typically >85% sleep efficiency, those with severe insomnia often have sleep efficiency <50%. Most patients will not describe daytime naps and there is often a waxing waning pattern with occasional nights of more normal sleep. Documented daytime naps should be infrequent or non-existent.
Additional screening questionnaires—which scales to use?
The Epworth Sleepiness Scale (ESS) is familiar to many in sleep clinics to assess daytime sleepiness (27). The typical insomnia patient should have a low normal score of 0–2. Advantages include speed and simplicity for the patient despite poorly correlating to more objective measures of sleep in the laboratory (28), we expect most of our patients to have low scores, and those scoring >10 should prompt screening for a secondary cause of insomnia.
The Insomnia Severity Index (ISI) is more often used within insomnia clinics and research studies and is validated to assess both severity and improvement after therapy with scores ranging from 0–24 (29) (>14 for moderate to severe insomnia). It is quick and easy to complete but will not distinguish between primary or secondary causes of insomnia.
CBTi as treatment of insomnia
The latest guidelines from the European Sleep Research Society and the American Academy of Sleep Medicine have all performed systematic reviews of relevant meta-analyses and concluded that CBTi should be the first line therapy offered to patients (30,31).
CBTi consists of a specific set of techniques that strengthen the bed-sleep connection, realign the homeostatic mechanisms and the circadian rhythm and decrease anxiety and rumination about sleep.
Typically therapy includes a combination of: psychoeducation about sleep and sleep hygiene, sleep restriction therapy, stimulus control therapy and cognitive therapy to decrease rumination about sleep as well as relaxation training. It is typically delivered over an average of six weekly sessions with most of the published manuals describing a need for 4–8 hours of therapy alongside weekly sleep diaries. The diaries are kept throughout therapy and help patient and treating therapist to see improvement and to personalize the behavioural components of bed and wake times.
The techniques are outlined in brief below.
Sleep hygiene/psychoeducation about sleep
Psychoeducation includes ‘sleep hygiene’. This is an explanation of the role of caffeine, alcohol and nicotine as sleep fragmenting lifestyle behaviours. The role of light and exercise in maintaining daytime alertness and the role of dark in the bedroom and the need for quiet is reviewed. Clock watching as an unhelpful bedtime behavior is explained but what tends to be key, is describing the normal sleep/wake cycle and age related changes. Many patients are genuinely surprised to understand that nobody “sleeps through” the night and the fact that waking during the night is normal. In the authors’ experience, the explanation of the role of light (outside daily whatever else is being done) and the effect of exercise to improve daytime alertness and night time sleep quality is often key. Exercise was not expanded upon in detail in the original CBTi regimes but more recent research has highlighted positive impacts on sleep (32). Being prescriptive about 30 minutes of moderate to high intensity exercise seems simple and effective adjuvant therapy that warrants further research but has a wealth of basic science and public health research to support its inclusion.
Relaxation techniques
This largely focuses upon the techniques of progressive muscle relaxation (a well-established method to decrease somatic tension by clenching and then releasing muscles in an orderly sequence moving down from top to toe over minutes) and simple imagery techniques to decrease a racing mind. In principle something a little more complex than counting sheep such as subtraction sums, detailed visual imagery or a version of simple alphabet games. Patients practice in the day and then use the techniques if it is difficult to fall asleep or if they wake.
Behavioural strategies
These techniques are undoubtedly difficult but considered highly effective including as stand-alone therapy (15,33). Using the sleep diary—patients restrict time in bed to achieve high sleep efficiency, therefore if someone records an average sleep time of 4.75 hours, then they have 5 hours in the bed room. The simple but fixed rules include:
- fixing wake time to the same time 7 days a week;
- get out of bed if unable to sleep (the quarter hour rule—i.e., no long periods awake and frustrated);
- no daytime napping;
- bedroom only for sleeping/sex;
- only getting into bed when very sleepy.
Driving safety needs to be considered and discussed and it is not recommended to sleep restrict for long periods, without support or review, in those with prior mania or for more than 2–3 weeks before gradually allowing increased time in bed again.
Cognitive therapy
These are psychological strategies to decrease any rumination prior to bed to change catastrophic thinking and beliefs about sleep and to reduce excessive worry and monitoring about sleep itself.
How to deliver CBTi therapy
Initial randomized controlled trial (RCT) studies provided evidence for benefit in individual or small group face to face therapy treating patients with insomnia disorder alone without comorbidities. Most studies concluded that somewhere between 4–8 sessions was effective averaging 6 hours of therapist time. The meta-analyses of these studies confirmed a large effect size both on completion of therapy but also stability of sleep over long term follow up [for review of RCTs to date 30].
Possibly of more relevance to general medicine is understanding that one can treat comorbid insomnia effectively with CBTi. A wide variety of co-morbid conditions were assessed with a recent large meta-analysis (34) and showed continued large effect sizes for the insomnia with modest benefits on the co-morbid conditions including chronic pain and depression.
In practice, this translates into 70–80% of patients within RCTs reporting a meaningful improvement in sleep efficiency and decreased insomnia severity at completion of therapy. Follow up studies suggest that 40–60% of people maintain these benefits over long term follow up.
There is more limited evidence for briefer versions of the therapy for example using just two face to face sessions (35) and two telephone calls or a single session and self-help material (36). There are now also several published self-help manuals available (37).
A number of computerized versions of CBTi have been developed in the US and UK such SHUTi, Sleepio and Sleepstation. The most recent meta-analyses of computerised CBTi show similar benefit to face to face CBTi (38), There were smaller effect sizes in an earlier meta-analysis published in 2012 (39). This may simply reflect increasingly sophisticated use of internet technology with the ability to incorporate more personalized and interactive systems. Possibly unsurprisingly—internet CBTi that incorporates a personalized feedback element had higher success rates (39). The health economic effects for internet based CBT have been explored given far lower costs and convenience for the patient (40). This is an increasingly standard method of delivery across health care systems and seems likely to increase in the coming years. Challenges will be ensuring safe screening for other sleep disorders and ensuring patient choice for those who need a higher intensity therapy. CBTi may be particularly appropriate as a computerized therapy given that the key measurement of sleep efficiency within sleep diaries can be easily digitalized.
The role of drugs—should patients have CBTi and medication?
At present, the hypnotics licensed for use are approved for short term therapy but in practice often used long term or issued on multiple occasions as for many insomnia is a chronic problem. In reality, hypnotic prescribing remains common in the UK and elsewhere because of a lack of knowledge of CBTi as an available therapy and because many patients essentially present in crisis or with acute distress, despite a chronic problem. Despite an initial fall in the high rates of long term prescriptions for hypnotics in the 1980s and 90s, prescribing patterns have been relatively stable over the last 10 years based on UK government prescribing data (9). Reviews of the effects of benzodiazepine and the Z drugs show very modest effects on sleep and a high rate of adverse events, particularly in the elderly (41). However there is some evidence that a combination of hypnotic and CBTi has greater benefit than CBTi alone when treatments were studied in combination (42,43) but during maintenance, discontinuation gave the best outcome in these studies where 68% of patients were in remission at 6 months. While many physicians perceive intermittent hypnotic use to allow fewer side effects and longer tern benefit, there is really no RCT data to support this view at present.
Sedating antidepressants have very weak evidence for benefit and tolerance develops quickly, as it does for antihistamines which are not recommended in any of the current guidelines for chronic insomnia (31).
Conclusions
- Insomnia disorder is common, is a risk factor for subsequent depression and warrants treatment. There are many mimics and a careful history and sleep diaries are needed for diagnosis.
- CBT for insomnia is an effective and well established therapy. This is now the recommended first line therapy so future challenges include increasing awareness about CBTi and increasing access for patients.
- With a sleep diary—a combination of behavioural measures, relaxation strategies, education about sleep and cognitive techniques significantly improve sleep and can be delivered either face to face in small groups, by a range of different health care professionals, online or with self-help. Delivery is effective with primary or secondary care settings.
- CBTi outperforms hypnotic medication and has fewer side effects but there is some evidence that if medication is used, then using it at the start of a CBTi programme and then weaning produces the best long term benefits to sleep.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Borbély AA, Daan S, Wirz-Justice A, et al. The two-process model of sleep regulation: a reappraisal. J Sleep Res 2016;25:131-43. [Crossref] [PubMed]
- Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004;27:1255-73. [Crossref] [PubMed]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med 2007;8:602-12. [Crossref] [PubMed]
- Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med 2014;30:591-627. [Crossref] [PubMed]
- Liu Y, Wheaton AG, Chapman DP, et al. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65:137-41. [Crossref] [PubMed]
- Roth T. New developments for treating sleep disorders. J Clin Psychiatry 2001;62:3-4. [PubMed]
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med 2006;7:123-30. [Crossref] [PubMed]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002;6:97-111. [Crossref] [PubMed]
- Calem M, Bisia J, Begum A, et al. Increased prevalence of insomnia and changes in hypnotic use in England over 15 years: analysis of the 1993, 2000 and 2007 national psychiatric morbidity surveys. Sleep 2012;35:377-84. [Crossref] [PubMed]
- International Classification of Sleep Disorders. 3rd edition. Darien, IL: American Academy of Sleep Medicine, 2014.
- DSM5. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association, Washington, 2013.
- Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med 2007;8 Suppl 4:S15-20. [Crossref] [PubMed]
- Baglioni C, Battagliese G, Feige B., et al. Insomnia as a predictor of depression, a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011;135:10-9. [Crossref] [PubMed]
- Ellis JG, Gerhman P, Espie CA, et al. The natural history of insomnia; focus on prevalence and incidence of acute insomnia. J Psychiatr Res 2012;46:1278-85. [Crossref] [PubMed]
- Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 1987;10:541-53. [PubMed]
- Espie CA, Broomfield NM, MacMahon KM, et al. The attention-effort pathway in the development of psychophysiological insomnia: a theoretical review. Sleep Med Rev 2006;10:215-45. [Crossref] [PubMed]
- Baglioni C, Nanovska S, Regen W, et al. Sleep changes in the disorder of insomnia; a meta-analysis of polysomnographic studies. Sleep Med Rev 2014;18:195-213. [Crossref] [PubMed]
- Ghosh D, Jamson SL, Baxter PD, et al. Continuous measures of driving performance on an advanced office-based driving simulator can be used to predict simulator task failure in patients with obstructive sleep apnoea syndrome. Thorax 2012;67:815-21. [Crossref] [PubMed]
- Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med 2014;15:860-73. [Crossref] [PubMed]
- Ferri R, Lanuzza B, Cosentino FI, et al. A single question for the rapid screening of restless legs syndrome in the neurological clinical practice. Eur J Neurol 2007;14:1016-21. [Crossref] [PubMed]
- Lichstein KL, Justin Thomas S, Woosley JA, et al. Co-occurring insomnia and obstructive sleep apnea. Sleep Med 2013;14:824-9. [Crossref] [PubMed]
- Abbott SM, Reid KJ, Zee PC. Circadian Rhythm Sleep-Wake Disorders. Psychiatr Clin North Am 2015;38:805-23. [Crossref] [PubMed]
- Gradisar M, Crowley SJ. Delayed sleep phase disorder in youth. Curr Opin Psychiatry 2013;26:580-5. [Crossref] [PubMed]
- Esquirol Y, Bongard V, Mabile L, et al. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int 2009;26:544-59. [Crossref] [PubMed]
- Fekedulegn D, Burchfiel CM, Hartley TA, et al. Shiftwork and sickness absence among police officers: the BCOPS stud16. Chronobiol Int 2013;30:930-41. [Crossref] [PubMed]
- Mantua J, Gravel N, Spencer RM. Reliability of Sleep Measures from Four Personal Health Monitoring Devices Compared to Research-Based Actigraphy and Polysomnography. Sensors (Basel) 2016;16:E646. [Crossref] [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [Crossref] [PubMed]
- Fong SY, Ho CK, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J Psychosom Res 2005;58:55-60. [Crossref] [PubMed]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297-307. [Crossref] [PubMed]
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675-700. [Crossref] [PubMed]
- Qaseem A, Kansagara D, Forciea MA, et al. Management of Chronic Insomnia Disorder in adults; a clinical practice guideline from the American college of physicians. Ann Intern Med 2016;165:125-33. [Crossref] [PubMed]
- Kredlow MA, Capozzoli MC, Hearon BA, et al. The effects of physical activity on sleep; a meta-analytic review. J Behav Med 2015;38:427-49. [Crossref] [PubMed]
- Miller CB, Espie CA, Epstein DR, et al. The evidence base of sleep restriction for treating insomnia disorder. Sleep Med Rev 2014;18:415-24. [Crossref] [PubMed]
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioural therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev 2015;23:54-67. [Crossref] [PubMed]
- Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment of chronic insomnia in older adults. Arch Intern Med 2011;171:887-95. [Crossref] [PubMed]
- Ellis JG, Cushing T, Germain A. Treating acute insomnia: a randomized controlled trial of a “single-shot” of cognitive behavioural therapy for insomnia. Sleep 2015;38:971-8. [PubMed]
- Espie CA. Overcoming insomnia and other sleep problems. London: Robinson, 2006.
- Zachariae R, Lyby MS, Ritterband LM, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia – a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30:1-10. [Crossref] [PubMed]
- Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom 2012;81:206-16. [Crossref] [PubMed]
- Thiart H, Ebert D, Lehr D, et al. Internet based cognitive behavioural therapy for insomnia, a health economic evaluation. Sleep 2016;39:1769-78. [Crossref] [PubMed]
- Glass J, Lanctot KL, Hermann N, et al. Sedative hypnotics in the elderly: meta-analysis of risks and benefits. BMJ 2005;331:1169. [Crossref] [PubMed]
- Morin CM, Colecchi C, Stone J, et al. Behavioural and pharmacological therapies for late life insomnia, a randomised controlled trial. JAMA 1999;281:991-9. [Crossref] [PubMed]
- Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA 2009;301:2005-15. [Crossref] [PubMed]


